 | |
| Clinical data | |
|---|---|
| Trade names | Almirid, Cripar |
| Other names | DHEC; 12'-Hydroxy-2'-(1-methylethyl)-5'α-(2-methylpropyl)-9,10α-dihydroergotaman-3',6',18-trione; (5'α,10α)-9,10-Dihydro-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-ergotaman-3',6',18-trione |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 12–16 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.706 |
| Chemical and physical data | |
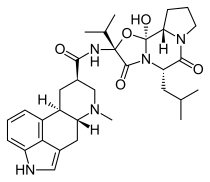
| Formula | C32H43N5O5 |
| Molar mass | 577.726 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Dihydroergocryptine (DHEC), sold under the brand names Almirid and Cripar among others, is a dopamine agonist of the ergoline group that is used as an antiparkinson agent in the treatment of Parkinson's disease.[1] It is taken by mouth.[citation needed]
- ^ Battistin L, Bardin PG, Ferro-Milone F, Ravenna C, Toso V, Reboldi G (January 1999). "Alpha-dihydroergocryptine in Parkinson's disease: a multicentre randomized double blind parallel group study". Acta Neurologica Scandinavica. 99 (1): 36–42. doi:10.1111/j.1600-0404.1999.tb00655.x. PMID 9925236. S2CID 45192184.