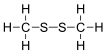| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Methyldisulfanyl)methane[1] | |||
| Other names
Dimethyl disulfide[1]
Methyl disulfide Methyldisulfide Dimethyldisulfide Methyldithiomethane 2,3-Dithiabutane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | DMDS | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.009.883 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
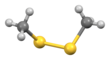
| CH3SSCH3 | |||
| Molar mass | 94.19 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.06 g/cm3[2] | ||
| Melting point | −85 °C (−121 °F; 188 K)[2] | ||
| Boiling point | 110 °C (230 °F; 383 K)[2] | ||
| 2.5 g/L (20 °C)[2] | |||
| Vapor pressure | 3.8 kPa (at 25 °C) Arkema data sheet | ||
| Hazards | |||
| Flash point | 15 °C (59 °F; 288 K)[2] | ||
| 370 °C (698 °F; 643 K)[2] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
190 mg/kg (oral, rat)[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dimethyl disulfide (DMDS) is an organic chemical compound with the molecular formula CH3SSCH3. It is a flammable liquid with an unpleasant, garlic-like odor. The compound is colorless although impure samples often appear yellowish.
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 708. doi:10.1039/9781849733069-FP001 (inactive 2024-06-30). ISBN 978-0-85404-182-4.
{{cite book}}: CS1 maint: DOI inactive as of June 2024 (link) - ^ a b c d e f Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ [1], EPA DMDS Fact Sheet