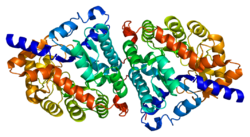
Dipeptidase 1 (DPEP1), or renal dipeptidase, is a membrane-bound glycoprotein responsible for hydrolyzing dipeptides. It is found in the microsomal fraction of the porcine kidney cortex.[5] It exists as a disulfide-linked homodimer that is glygosylphosphatidylinositol (GPI)-anchored to the renal brush border of the kidney.[6] The active site on each homodimer is made up of a barrel subunit with binuclear zinc ions that are bridged by the Gly125 side-chain located at the bottom of the barrel.[7]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000015413 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000019278 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Armstrong, David J., Sunil K. Mukhopadhyay, and Benedict J. Campbell. "Physicochemical characterization of renal dipeptidase." Biochemistry 13.8 (1974): 1745-750. Web.
- ^ Keynan, Shoshana, Nicolette T. Habgood, Nigel M. Hooper, and Anthony J. Turner. "Site-Directed Mutagenesis of Conserved Cysteine Residues in Porcine Membrane Dipeptidase. Cys 361 Alone Is Involved in Disulfide-Linked Dimerization†." Biochemistry 35.38 (1996): 12511-2517. Web.
- ^ Nitanai, Yasushi, Yoshinori Satow, Hideki Adachi, and Masafumi Tsujimoto. "Crystal Structure of Human Renal Dipeptidase Involved in β-Lactam Hydrolysis." Journal of Molecular Biology 321.2 (2002): 177-84. Web.