 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksəˈruːbɪsɪn/ |
| Trade names | Adriamycin, Caelyx,[1] Myocet,[2] others |
| Biosimilars | Zolsketil pegylated liposomal,[3] Celdoxome pegylated liposomal[4] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682221 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | intravenous, intravesical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (by mouth) |
| Protein binding | 75%[8] |
| Metabolism | Liver |
| Elimination half-life | Triphasic; 12 minutes, 3.3 hours, 30 hours. Mean: 1–3 hours[8][9] |
| Excretion | Urine (5–12%), faeces (40–50%)[8] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.344 |
| Chemical and physical data | |
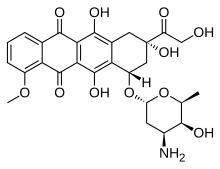
| Formula | C27H29NO11 |
| Molar mass | 543.525 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer.[10] This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia.[10] It is often used together with other chemotherapy agents.[10] Doxorubicin is given by injection into a vein.[10]
Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth.[10] Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia.[10] People often experience red discoloration of the urine for a few days.[10] Doxorubicin is in the anthracycline and antitumor antibiotic family of medications.[10] It works in part by interfering with the function of DNA.[11]
Doxorubicin was approved for medical use in the United States in 1974.[10] It is on the World Health Organization's List of Essential Medicines.[12][13] Versions that are pegylated and in liposomes are also available; however, they are more expensive.[13] Doxorubicin was originally made from the bacterium Streptomyces peucetius.[14]
- ^ a b "Caelyx pegylated liposomal EPAR". European Medicines Agency (EPAR). 17 September 2018. Archived from the original on 21 June 2022. Retrieved 20 June 2022.
- ^ a b Cite error: The named reference
Myocet liposomal EPARwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Zolsketil pegylated liposomal EPARwas invoked but never defined (see the help page). - ^ a b "Celdoxome pegylated liposomal EPAR". European Medicines Agency (EMA). 20 June 2022. Retrieved 31 January 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Caelyx product information". Health Canada. 25 April 2012. Archived from the original on 21 June 2022. Retrieved 20 June 2022.
- ^ "Myocet product information". Health Canada. 25 April 2012. Retrieved 20 June 2022.
- ^ "Taro-doxorubicin liposomal product information". Health Canada. 25 April 2012. Archived from the original on 14 May 2021. Retrieved 20 June 2022.
- ^ a b c "(doxorubicin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 16 April 2014. Retrieved 15 April 2014.
- ^ Brayfield A, ed. (19 December 2013). "Doxorubicin". Martindale: The Complete Drug Reference. Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 15 April 2014.
- ^ a b c d e f g h i "Doxorubicin Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 11 October 2016. Retrieved 12 January 2017.
- ^ Tacar O, Sriamornsak P, Dass CR (February 2013). "Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems". The Journal of Pharmacy and Pharmacology. 65 (2): 157–170. doi:10.1111/j.2042-7158.2012.01567.x. PMID 23278683. S2CID 34737360.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 583. ISBN 9780857111562.
- ^ Ravina E (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 291. ISBN 9783527326693. Archived from the original on 18 September 2017.