This article needs additional citations for verification. (June 2015) |
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Viberzi (/vaɪˈbɜːrzi/ vy-BUR-zee |
| Trade names | Viberzi, Truberzi |
| Other names | JNJ-27018966 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 81% |
| Elimination half-life | 3.7–6 hours |
| Excretion | 82.2% (feces), <1% (urine)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
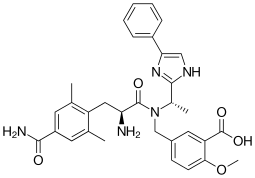
| Formula | C32H35N5O5 |
| Molar mass | 569.662 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eluxadoline, sold under the brand names Viberzi and Truberzi,[3] is a medication taken by mouth for the treatment of diarrhea and abdominal pain in individuals with diarrhea-predominant irritable bowel syndrome (IBS-D).[4] It was approved for use in the United States in 2015.[5] The drug originated from Janssen Pharmaceutica and was developed by Actavis.
- ^ "Digestive and bladder health". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ a b "Viberzi- eluxadoline tablet, film coated". DailyMed. 24 July 2024. Retrieved 11 November 2024.
- ^ "Truberzi". European Medicines Agency. 29 September 2016. Archived from the original on 21 September 2017. Retrieved 28 June 2017.
- ^ Fragkos KC (2017-09-25). "Spotlight on eluxadoline for the treatment of patients with irritable bowel syndrome with diarrhea". Clinical and Experimental Gastroenterology. 10: 229–240. doi:10.2147/ceg.s123621. PMC 5624596. PMID 28989282.
- ^ "FDA approves two therapies to treat IBS-D". www.fda.gov. Retrieved 2015-06-01.[dead link]