 | |
| Clinical data | |
|---|---|
| Trade names | Jardiance, others |
| Other names | BI-10773 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614043 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sodium-glucose cotransporter-2 (SGLT2) inhibitor[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.122.058 |
| Chemical and physical data | |
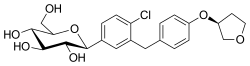
| Formula | C23H27ClO7 |
| Molar mass | 450.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Empagliflozin, sold under the brand name Jardiance, among others, is an antidiabetic medication used to improve glucose control in people with type 2 diabetes.[12][2][14] It is taken by mouth.[2]
Common side effects include hyperventilation, anorexia, abdominal pain, nausea, vomiting, lethargy, mental status changes, hypotension, acute kidney injury, and vaginal yeast infections.[2] Rarer but more serious side effects include a skin infection of the groin called Fournier's gangrene and a form of diabetic ketoacidosis with normal blood sugar levels.[2][15] Use during pregnancy or breastfeeding is not recommended.[16] Empagliflozin sometimes causes a transient decline in kidney function, and on rare occasions causes acute kidney injury, so use should be monitored in those with kidney dysfunction. But some trials have indicated that empagliflozin can be used in people with an eGFR as low as 20 mL/min/1.73 m², without increasing adverse kidney outcomes.[17][18]
The use of empagliflozin has been shown to improve outcomes in people with established cardiovascular disease.[19][17] There is evidence from high quality studies that empagliflozin can also help to slow the rate of kidney function decline. Irrespective of diabetes status, benefit was observed in those with mild, moderate or severe loss of kidney function.[20][21] People started on empagliflozin may first see a decrease in kidney function before their glomerular filtration rate stabilises.[22] Greatest benefit was demonstrated in those who had severe loss of kidney function, higher risk of kidney function worsening and background of diabetes.[20]
Empagliflozin is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), and works by increasing sugar loss in urine.[2]
Empagliflozin was approved for medical use in the United States and in the European Union in 2014.[13][23][24] It is on the World Health Organization's List of Essential Medicines.[25] In 2022, it was the 56th most commonly prescribed medication in the United States, with more than 12 million prescriptions.[26][27] It has received approval as a generic medication from the US Food and Drug Administration (FDA).[28]
- ^ "Empagliflozin (Jardiance) Use During Pregnancy". Drugs.com. 30 August 2018. Archived from the original on 5 August 2019. Retrieved 10 February 2020.
- ^ a b c d e f "Empagliflozin Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 6 April 2019. Retrieved 21 December 2018.
- ^ "AusPAR: Empagliflozin". Therapeutic Goods Administration (TGA), Commonwealth of Australia. 8 November 2017. Retrieved 24 March 2022.
- ^ "Jardiance". Boehringer Ingelheim Pty Ltd. Therapeutic Goods Administration (TGA), Commonwealth of Australia. Archived from the original on 18 March 2023.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA), Commonwealth of Australia. 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ "Prescription medicines and biologicals: TGA annual summary 2017". Therapeutic Goods Administration (TGA), Commonwealth of Australia. 21 June 2022. Retrieved 31 March 2024.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ "Jardiance Product information". Health Canada. 11 August 2015. Retrieved 8 September 2024.
- ^ "Jardiance Product information". Health Canada. 11 August 2015. Retrieved 8 September 2024.
- ^ "Jardiance 10 mg film-coated tablets – Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 20 September 2020. Retrieved 10 February 2020.
- ^ "Jardiance 25 mg film-coated tablets – Summary of Product Characteristics (SmPC)". (emc). 23 October 2019. Archived from the original on 22 September 2020. Retrieved 10 February 2020.
- ^ a b Cite error: The named reference
Jardiance FDA labelwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Jardiance EPARwas invoked but never defined (see the help page). - ^ Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. (December 2018). "Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)". Diabetologia. 61 (12): 2461–2498. doi:10.1007/s00125-018-4729-5. PMID 30288571.
- ^ "FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes". U.S. Food and Drug Administration (FDA). 9 February 2019. Archived from the original on 13 December 2019. Retrieved 18 March 2019.
- ^ British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 691. ISBN 9780857113382.
- ^ a b Zannad F, Butler J, Filippatos GS, Pocock S, Jamal W, Schnee J, et al. (May 2021). "Cardiovascular and Kidney Outcomes with Empagliflozin in Heart Failure". Präzisionsmedizin – Eine Reise in die Zukunft der Diabetologie www.diabeteskongress.de. 16. Georg Thieme Verlag KG. doi:10.1055/s-0041-1727471.
- ^ Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al. (January 2023). "Empagliflozin in Patients with Chronic Kidney Disease". The New England Journal of Medicine. 388 (2): 117–127. doi:10.1056/NEJMoa2204233. PMC 7614055. PMID 36331190.
- ^ Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. (November 2015). "Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes". The New England Journal of Medicine. 373 (22): 2117–2128. doi:10.1056/NEJMoa1504720. PMID 26378978.
- ^ a b Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, et al. (August 2020). "Erratum: The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study". Clinical Kidney Journal. 13 (4): 722. doi:10.1056/NEJMoa2204233. hdl:20.500.13003/18576. PMC 7467589. PMID 32905262.
- ^ Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. (July 2016). "Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes". The New England Journal of Medicine. 375 (4): 323–334. doi:10.1056/nejmoa1515920. PMID 27299675.
- ^ Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. (July 2016). "Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes". The New England Journal of Medicine. 375 (4): 323–334. doi:10.1056/NEJMoa1515920. PMID 27299675.
- ^ Cite error: The named reference
FDA PR 20140801was invoked but never defined (see the help page). - ^ "Drug Approval Package: Jardiance (empagliflozin) Tablets NDA #204629". U.S. Food and Drug Administration (FDA). 8 September 2014. Archived from the original on 11 February 2020. Retrieved 10 February 2020.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Empagliflozin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "2022 First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). 3 March 2023. Archived from the original on 14 January 2024. Retrieved 14 January 2024.