 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
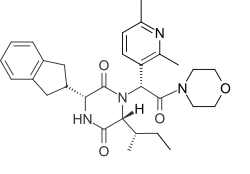
| Formula | C30H38N4O4 |
| Molar mass | 518.658 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Epelsiban (INN,[1] USAN,[2] code name GSK-557,296-B) is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist (Ki = 0.13 nM).[3][4] It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men[5][6] and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF).,[7] and was also investigated for use in the treatment of adenomyosis.[8]
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 67" (PDF). World Health Organization. p. 62. Archived from the original (PDF) on October 5, 2016. Retrieved 4 October 2016.
- ^ USAN Council (2011). "Statement on a Nonproprietary Name Adopted by the USAN Council" (PDF). Retrieved 2011-10-28.
- ^ Borthwick AD, Liddle J, Davies DE, Exall AM, Hamlett C, Hickey DM, Mason AM, Smith IE, Nerozzi F, Peace S, Pollard D, Sollis SL, Allen MJ, Woollard PM, Pullen MA, Westfall TD, Stanislaus DJ (January 2012). "Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency". Journal of Medicinal Chemistry. 55 (2): 783–96. doi:10.1021/jm201287w. PMID 22239250.
- ^ Borthwick AD, Liddle J (January 2013). "Retosiban and Epelsiban: Potent and Selective Orally available Oxytocin Antagonists". In Domling A (ed.). Methods and Principles in Medicinal Chemistry: Protein-Protein Interactions in Drug Discovery. Weinheim: Wiley-VCH. pp. 225–256. doi:10.1002/9783527648207.ch10. ISBN 978-3-527-33107-9.
- ^ Clément P, Bernabé J, Compagnie S, Alexandre L, McCallum S, Giuliano F (August 2013). "Inhibition of ejaculation by the non‐peptide oxytocin receptor antagonist GSK 557296: a multi‐level site of action". British Journal of Pharmacology. 169 (7): 1477–1485. doi:10.1111/bph.12198. PMC 3724105. PMID 23530818.
- ^ Shinghal R, Barnes A, Mahar KM, Stier B, Giancaterino L, Condreay LD, Black L, McCallum SW (October 2013). "Safety and efficacy of epelsiban in the treatment of men with premature ejaculation: A randomized, double‐blind, placebo‐controlled, fixed‐dose study". The Journal of Sexual Medicine. 10 (10): 2506–2517. doi:10.1111/jsm.12272. PMID 23937679.
- ^ Mahar KM, Stier B, Fries M, McCallum SW (November 2015). "A single- and multiple-dose study to investigate the pharmacokinetics of epelsiban and its metabolite, GSK2395448, in healthy female volunteers". Clinical Pharmacology in Drug Development. 4 (6): 418–426. doi:10.1002/cpdd.210. PMID 27137713. S2CID 23903528.
- ^ Mahar KM, Enslin MB, Gress A, Amrine-Madsen H, Cooper M (January 2018). "Single‐and Multiple‐Day Dosing Studies to Investigate High‐Dose Pharmacokinetics of Epelsiban and Its Metabolite, GSK2395448, in Healthy Female Volunteers". Clinical Pharmacology in Drug Development. 7 (1): 33–43. doi:10.1002/cpdd.363. PMID 28556598. S2CID 41083332.