 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛpˈlɛrənoʊn/ |
| Trade names | Inspra, others |
| Other names | SC-66110; CGP-30083; 9-11α-Epoxymexrenone; 9,11α-Epoxy-7α-methoxycarbonyl-3-oxo-17α-pregn-4-ene-21,17-carbolactone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603004 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~70%[2] |
| Protein binding | ~50% (33–60%) (primarily to α1-acid glycoprotein)[2][3] |
| Metabolism | Liver (CYP3A4)[2][3] |
| Metabolites | 6β-OH-EPL, 6β,21-OH-EPL, 21-OH-EPL, 3α,6β-OH-EPL[2] (All inactive)[2] |
| Elimination half-life | 4–6 hours[4] |
| Excretion | Urine (67%), feces (32%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.615 |
| Chemical and physical data | |
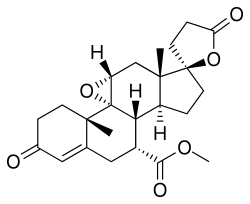
| Formula | C24H30O6 |
| Molar mass | 414.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for people with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA).[6]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 743–. ISBN 978-1-60913-345-0.
- ^ a b Sica DA (January 2005). "Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis". Heart Failure Reviews. 10 (1): 23–9. doi:10.1007/s10741-005-2345-1. PMID 15947888. S2CID 21437788.
- ^ Struthers A, Krum H, Williams GH (April 2008). "A comparison of the aldosterone-blocking agents eplerenone and spironolactone". Clinical Cardiology. 31 (4): 153–8. doi:10.1002/clc.20324. PMC 6652937. PMID 18404673.
- ^ Frishman WH, Cheng-Lai A, Nawarskas J (4 January 2005). Current Cardiovascular Drugs. Springer Science & Business Media. pp. 246–. ISBN 978-1-57340-221-7.
- ^ Delyani JA, Rocha R, Cook CS, Tobert DS, Levin S, Roniker B, et al. (2001). "Eplerenone: a selective aldosterone receptor antagonist (SARA)". Cardiovascular Drug Reviews. 19 (3): 185–200. doi:10.1111/j.1527-3466.2001.tb00064.x. PMID 11607037.
{{cite journal}}: CS1 maint: overridden setting (link)