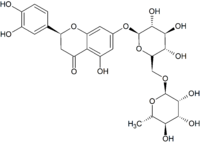
| |
| Names | |
|---|---|
| IUPAC name
(2S)-3′,4′,5-Trihydroxy-7-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavan-4-one
| |
| Systematic IUPAC name
(22S,42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-13,14,25,43,44,45,73,74,75-Nonahydroxy-76-methyl-22,23-dihydro-24H-3,6-dioxa-2(2,7)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-24-one | |
| Other names
Eriodictyol glycoside
Eriodictyol-7-O-rutinoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.321 |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H32O15 | |
| Molar mass | 596.538 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eriocitrin (eriodictyol glycoside) is a flavanone-7-O-glycoside between the flavanone eriodictyol and the disaccharide rutinose. It is commonly found in lemons and other citrus fruits.[1][2][3][4] It is colloquially called lemon flavonoid or a citrus flavonoid, one of the plant pigments that bring color to fruit and flowers. This antioxidant also predominates (38% in 1 study) in Peppermint infusions.
The compound has lipid-lowering properties in liver cells.[5] It is marketed as a dietary supplement, usually in conjunction with B and C vitamins and other substances, but there is no established medical use or FDA approved application of the compound.
- ^ Cao X, He Y, Kong Y, Mei X, Huo Y, He Y, Liu J (September 2019). "Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods". Food Hydrocolloids. 94: 63–70. doi:10.1016/j.foodhyd.2019.03.006. ISSN 0268-005X. S2CID 107490400.
- ^ Miyake Y, Suzuki E, Ohya S, Fukumoto S, Hiramitsu M, Sakaida K, Osawa T, Furuichi Y (2006-11-13). "Lipid-Lowering Effect of Eriocitrin, the Main Flavonoid in Lemon Fruit, in Rats on a High-Fat and High-Cholesterol Diet". Journal of Food Science. 71 (9): S633–S637. doi:10.1111/j.1750-3841.2006.00192.x. ISSN 0022-1147.
- ^ Miyake Y, Yamamoto K, Morimitsu Y, Osawa T (1997-12-01). "Isolation of C -Glucosylflavone from Lemon Peel and Antioxidative Activity of Flavonoid Compounds in Lemon Fruit". Journal of Agricultural and Food Chemistry. 45 (12): 4619–4623. doi:10.1021/jf970498x. ISSN 0021-8561.
- ^ Miyake Y, Yamamoto K, Osawa T (1997). "Isolation of Eriocitrin (Eriodictyol 7-rutinoside) from Lemon Fruit (Citrus limon Burm. f.) and Its Antioxidative Activity". Food Science and Technology International, Tokyo. 3 (1): 84–89. doi:10.3136/fsti9596t9798.3.84. ISSN 1881-3976.
- ^ Hiramitsu M, Shimada Y, Kuroyanagi J, Inoue T, Katagiri T, Zang L, et al. (January 2014). "Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis". Scientific Reports. 4: 3708. Bibcode:2014NatSR...4E3708H. doi:10.1038/srep03708. PMC 3892443. PMID 24424211.