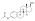 | |
| Clinical data | |
|---|---|
| Trade names | Depofollan |
| Other names | E2-17-St; Estradiol octadecanoate; Estradiol 17β-stearate; Estradiol 17β-octadecanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C36H58O3 |
| Molar mass | 538.857 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
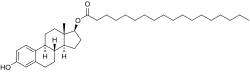
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol.[1][2][3][4][5] It occurs in the body as a very long-lasting metabolite and prohormone of estradiol.[5] The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate.[6][5] It is extremely lipophilic and hydrophobic.[5] Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity.[7][8][9][5] The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.[5]
Estradiol stearate has a prolonged duration of action relative to estradiol regardless of whether it is given by intravenous injection or subcutaneous injection.[7] This is in contrast to short-chain fatty acid esters of estradiol, such as estradiol benzoate, which do not show a prolonged duration with intravenous injection.[10] When administered by intravenous injection in rodents, estradiol stearate has a greatly increased terminal half-life relative to estradiol (6 hours vs. 2 minutes).[7] Estradiol stearate also had a half-life that was 60% longer than that of estradiol arachidonate, despite similar ester chain lengths.[7] In contrast to the long-chain esters, the half-lives of short-chain estradiol esters such as estradiol acetate and estradiol hexanoate were the same as that of estradiol.[7] As such, whereas short-chain estradiol esters are rapidly hydrolyzed, long-chain estradiol esters like estradiol stearate are resistant to metabolism.[7] Thus, the prolongation of effect of short-chain estradiol esters is purely due to their increased lipophilicity and slow release from the injected depot, whereas the prolonged duration of long-chain estradiol esters is due both to this property and to their resistance to metabolism.[7] Estradiol stearate is susceptible to first-pass metabolism in the liver, and hence has much greater potency by subcutaneous injection than by oral administration.[7]
In addition to its endogenous role, estradiol stearate was previously available as a pharmaceutical drug for use via depot intramuscular injection.[1][2] The medication was introduced between 1938 and 1941 under the brand name Depofollan.[11][12] It has been used to treat prostate cancer.[13][14] Estradiol stearate is a long-acting estrogen[15][12] and is said to have been the first long-acting estrogen used in medicine, although it was never widely employed.[12] It was reported to have a duration of more than one month.[12] The medication was provided as an oil solution in ampoules containing 15 mg estradiol stearate.[15][14] It was manufactured by Chinoin, a Hungarian pharmaceutical company.[15][14][11][16] The compound was studied by Karl Miescher in 1938[17] and was patented by Miescher and Chinoin in 1939 and 1941, respectively.[18][19] A similar clinically used long-acting estradiol ester is estradiol undecylate, which has 11 carbon atoms instead of the 18 carbon atoms in estradiol stearate.[1][2]
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
- ^ a b c Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 898. ISBN 978-1-4757-2085-3.
- ^ a b c Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 405–. ISBN 978-3-88763-075-1.
- ^ Negwer M (1987). Organic-chemical Drugs and Their Synonyms: (an International Survey). VCH Publishers. ISBN 978-0-89573-552-2.
Estra-1,3,5(10)-triene-3,173-diol 17-octadecanoate = 3,173-Estradiol 17-stearate = (173)-Estra-1,3,5- (10)-triene-3,17-diol 17-octadecanoate (e) S Depofollan, Estradiol stearate, Ostradiolstearat U Depot-estrogen 8103
- ^ Josephy E, Radt F (1956). Elsevier's Encyclopædia of Organic Chemistry. pp. 1974–1976.
- ^ a b c d e f Hochberg RB, Pahuja SL, Larner JM, Zielinski JE (1990). "Estradiol-fatty acid esters. Endogenous long-lived estrogens". Annals of the New York Academy of Sciences. 595 (1): 74–92. Bibcode:1990NYASA.595...74H. doi:10.1111/j.1749-6632.1990.tb34284.x. PMID 2197972. S2CID 19866729.
- ^ Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 235–237. ISBN 978-3-642-58616-3.
- ^ a b c d e f g h Hochberg RB (June 1998). "Biological esterification of steroids". Endocrine Reviews. 19 (3): 331–348. doi:10.1210/edrv.19.3.0330. PMID 9626557.
- ^ Janocko L, Larner JM, Hochberg RB (April 1984). "The interaction of C-17 esters of estradiol with the estrogen receptor". Endocrinology. 114 (4): 1180–1186. doi:10.1210/endo-114-4-1180. PMID 6705734.
- ^ Vazquez-Alcantara MA, Menjivar M, Garcia GA, Díaz-Zagoya JC, Garza-Flores J (December 1989). "Long-acting estrogenic responses of estradiol fatty acid esters". Journal of Steroid Biochemistry. 33 (6): 1111–1118. doi:10.1016/0022-4731(89)90417-2. PMID 2515394.
- ^ Parkes AS (February 1938). "Effective Absorption of Hormones". British Medical Journal. 1 (4024): 371–373. doi:10.1136/bmj.1.4024.371. PMC 2085798. PMID 20781252.
- ^ a b Antalné S, Géza B, István B, Dezső K. A Chinoin története (1910–1995) [The history of Chinoin] (PDF) (in Hungarian).
From the middle of the vitamin D and hannincas years, the research and production of steroid sex hormones was directed by Rezső Weisz. (n) In the latter, the collection of animal raw materials, in addition to chemical operations, required considerable organizational work similar to that of insulin. The intensification of attempts to produce steroid hormones and synthetic steroids, estrogens, over four years may have surpassed even 36 studies of sulfone JDid. Weisz et al., Primarily Kálmán Lányi, developed the most important estrone derivatives of industrial and therapeutic interest, Hogival (estrone acetate), Acrafalin (estradiol propionate) and Depofollan (estradiol stearate), which were marketed in 1938-1940.
- ^ a b c d Orvostudomány [Medicine] (in Hungarian). Magyar Tudomanyos Akadémia V. Orvosi Tudományok Osztálya (Hungarian Academy of Sciences V. Department of Medical Sciences). 1960. p. 11.
- ^ Medgyaszay A (1963). "[Intraocular pressure and hormone therapy]". Ophthalmologica. Journal International d'Ophtalmologie. International Journal of Ophthalmology. Zeitschrift Fur Augenheilkunde. 145 (3): 243–248. doi:10.1159/000304442. PMID 13934365.
- ^ a b c Sellei C, Eckhardt S, Németh L (1970). Chemotherapy of Neoplastic Diseases. Budapest: Akadémiai Kiadó. p. 251.
- ^ a b c Endokrinologie. Johann Ambrosius Barth Verlag. 1951.
- ^ Gyár R, Tiszavasvári T, Novák K, Hermecz I, eds. (2005). "Esti beszélgetés – Magyar Gyógyszerkutatók portréi." [Evening discussion - Portraits of Hungarian Pharmaceutical Researchers.]. Alkaloida Vegyészeti [Alkaloid Chemistry] (PDF) (in Hungarian). Budapest: MTA Gyógyszerkémiai és Gyógyszertechnológiai Munkabizottsága (Hungarian Academy of Sciences' Working Committee on Medicinal Chemistry and Pharmaceutical Technology). Archived from the original (PDF) on 2016-08-05.
Chinoin has achieved good results in the steroid hormones, synthetic steroids, and estrogens since the twenties, protected by 36 patents. The company was one of the first in the world to produce vitamin D, but was also a successful product for Hogival (estrone acetate), Acrofollin (estradiol propionate), Depofollan (estradiol stearate), Acrolutin (progesterone).
- ^ Miescher K, Scholz C, Tschopp E (August 1938). "The activation of female sex hormones: Mono-esters of alpha-oestradiol". The Biochemical Journal. 32 (8): 1273–1280. doi:10.1042/bj0321273b. PMC 1264184. PMID 16746750.
- ^ US 2156599, Karl M, Caesar S, "Estradiol esters esterified in 3-position and process of making same", issued 1939
- ^ US 2253669, Rezso W, "Estradiol higher fatty acid ester", issued 1941, assigned to Current Assignee Chinoin Private Co Ltd