| |
| Names | |
|---|---|
| IUPAC name
N,N′-(Ethane-1,2-diyl)bis[N-(carboxymethyl)glycine][1]
| |
| Systematic IUPAC name
2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | EDTA, H4EDTA |
| 1716295 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.409 |
| EC Number |
|
| 144943 | |
| KEGG | |
| MeSH | Edetic+Acid |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N2O8 | |
| Molar mass | 292.244 g·mol−1 |
| Appearance | Colourless crystals |
| Density | 0.860 g cm−3 (at 20 °C) |
| log P | −0.836 |
| Acidity (pKa) | 2.0, 2.7, 6.16, 10.26[2] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−1765.4 to −1758.0 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4461.7 to −4454.5 kJ mol−1 |
| Pharmacology | |
| S01XA05 (WHO) V03AB03 (WHO) (salt) | |
| |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1000 mg/kg (oral, rat)[3] |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
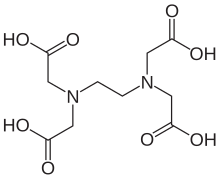
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.[4]
- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 79, 123, 586, 754. ISBN 978-0-85404-182-4.
- ^ Raaflaub, Jürg (1956). "Applications of Metal Buffers and Metal Indicators in Biochemistry". Methods of Biochemical Analysis. Vol. 3. pp. 301–325. doi:10.1002/9780470110195.ch10. ISBN 978-0-470-30492-1. PMID 13369167.
- ^ Substance Name: Sodium calcium edetate. NIH.gov
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).
