An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens.[1] Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration.[2] However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides).[3][4][5] As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.[6]
Utilization of exoelectrogens is currently being researched in the development of microbial fuel cells (MFCs), which hold the potential to convert organic material like activated sludge from waste water treatment into ethanol, hydrogen gas, and electric current.[1][7]
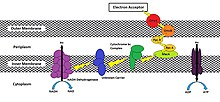
While the exact process in which a cell will reduce an extracellular acceptor will vary from species to species, methods have been shown to involve the use of an oxidoreductase pathway that will transport electrons to the cell membrane that is exposed to the external environment.[3] This pathway splits off from the ETC pathway after the cytochrome bc1 complex (Complex III) is oxidized by c-type cytochromes designed to move electrons towards the extracellular face of its outermost membrane instead of towards cytochrome c oxidase (Complex IV). MtrC and OmcA are examples of such c-type cytochromes that are endogenously found in the outer membrane of Shewanella oneidensis MR-1 a gammaproteobacterium, though many other variations exist (Figure 1).[3][4][5][7][8]
Aside from releasing electrons to an exogenous final electron acceptor, external electron transfer may serve other purposes. First, cells may transfer electrons directly to each other without the need for an intermediary substance. Pelotomaculum thermopropioncum has been observed linked to Methanothermobacter thermautotrophicus by a pilus (external cell structures used in conjugation and adhesion) that was determined to be electrically conductive. Second, extracellular electrons may serve a role in the communication as a quorum signal in biofilms.[1]
In addition to S. oneidensis MR-1, exoelectrogenic activity has been observed in the following strains of bacteria without an exogenous mediator: Shewanella putrefaciens IR-1, Clostridium butyricum, Desulfuromonas acetoxidans, Geobacter metallireducens, Geobacter sulfurreducens, Rhodoferax ferrireducens, Aeromonas hydrophilia (A3), Pseudomonas aeruginosa, Desulfobulbus propionicus, Geopsychrobacter electrodiphilus, Geothrix fermentans, Shewanella oneidensis DSP10, Escherichia coli, Rhodopseudomonas palustris, Brucella anthropi YZ-1, Desulfovibrio desulfuricans, Acidiphilium sp.3.2Sup5, Klebsiella pneumoniae L17, Thermincola sp.strain JR, Pichia anomala.[1]
- ^ a b c d Logan B. (May 2009). "Exoelectrogenic bacteria that power microbial fuel cells". Nature Reviews Microbiology. 7 (5): 375–383. doi:10.1038/nrmicro2113. PMID 19330018. S2CID 2560062.
- ^ Willey J.; et al. (2011). Prescott's Microbiology. McGraw Hill. pp. 228–245. ISBN 978-0-07-337526-7.
- ^ a b c Hartshorne R.; et al. (Dec 2009). "Characterization of an electron conduit between bacteria and the extracellular environment" (PDF). Proceedings of the National Academy of Sciences. 106 (52): 22169–22174. Bibcode:2009PNAS..10622169H. doi:10.1073/pnas.0900086106. PMC 2799772. PMID 20018742.
- ^ a b Baron D. (Oct 2009). "Electrochemical Measurement of Electron Transfer Kinetics by Shewanella oneidensis MR-1". The Journal of Biological Chemistry. 284 (42): 28865–28873. doi:10.1074/jbc.M109.043455. PMC 2781432. PMID 19661057.
- ^ a b Shi L.; et al. (2006). "Isolation of a High-Affinity Functional Protein Complex between OmcA and MtrC: Two Outer Membrane Decaheme c-Type Cytochromes of Shewanella oneidensis MR-1". Journal of Bacteriology. 188 (13): 4705–4714. doi:10.1128/JB.01966-05. PMC 1483021. PMID 16788180.
- ^ Logan B. (2008). Microbial Fuel Cells. John Wiley & Sons Inc. pp. 4–6. ISBN 978-0470239483.
- ^ a b Flynn J.; et al. (2010). "Enabling Unbalanced Fermentations by Using Engineered Electrode-Interfaced Bacteria". mBio. 1 (5): 1–8. doi:10.1128/mBio.00190-10. PMC 2975363. PMID 21060736.
- ^ Lovley D. (2008). "The microbe electric: conversion of organic matter to electricity". Current Opinion in Biotechnology. 19 (6): 1–8. doi:10.1016/j.copbio.2008.10.005. PMID 19000760.