 | |
| Clinical data | |
|---|---|
| Trade names | Felbatol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606011 |
| Routes of administration | By mouth (tablets, oral suspension) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Metabolism | Hepatic |
| Elimination half-life | 20–23 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.714 |
| Chemical and physical data | |
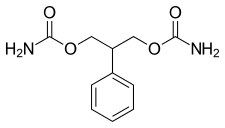
| Formula | C11H14N2O4 |
| Molar mass | 238.243 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Felbamate (marketed under the brand name Felbatol by MedPointe) is an anticonvulsant[2] used in the treatment of epilepsy. It is used to treat partial seizures[3][4] (with and without generalization) in adults and partial and generalized seizures associated with Lennox–Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drug's usage to severe refractory epilepsy.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Rho JM, Donevan SD, Rogawski MA (March 1997). "Barbiturate-Like Actions of the Propanediol Dicarbamates Felbamate and Meprobamate". J. Pharmacol. Exp. Ther. 280 (3): 1383–91. PMID 9067327.
- ^ Leppik IE, Dreifuss FE, Pledger GW, et al. (November 1991). "Felbamate for Partial Seizures: Results of a Controlled Clinical Trial". Neurology. 41 (11): 1785–9. doi:10.1212/wnl.41.11.1785. PMID 1944909. S2CID 25245002.
- ^ Devinsky O, Faught RE, Wilder BJ, et al. (March 1995). "Efficacy of Felbamate Monotherapy in Patients Undergoing Presurgical Evaluation of Partial Seizures". Epilepsy Res. 20 (3): 241–6. doi:10.1016/0920-1211(94)00084-A. PMID 7796796. S2CID 21915205.