 | |
| Clinical data | |
|---|---|
| Other names | CP-10188; Fenclonina; Fencloninum; NSC-77370; Parachlorophenylalanine[1] |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.229 |
| Chemical and physical data | |
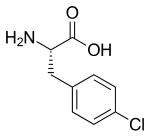
| Formula | C9H10ClNO2 |
| Molar mass | 199.63 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.336 g/cm3 |
| Melting point | 240 °C (464 °F) |
| Boiling point | 339.5 °C (643.1 °F) |
| |
| |
| (verify) | |
Fenclonine, also known as para-chlorophenylalanine (PCPA), acts as a selective and irreversible inhibitor of tryptophan hydroxylase, which is a rate-limiting enzyme in the biosynthesis of serotonin.[2]
It has been used experimentally to treat carcinoid syndrome, but the side effects, mostly hypersensitivity reactions and psychiatric disturbances, have prevented development for this use.[3]
The effects of serotonin depletion from fenclonine are so drastic that serotonin cannot even be detected immunohistochemically within the first day after administration of a control dose. Tryptophan hydroxylase activity can be detected neither in cell bodies or nerve terminals. After one week 10% of control values (the baseline extrapolated for the study) had replenished in the raphe nucleus, and after two weeks from initial treatment as much was again detected in the hypothalamus region. Aromatic L-amino acid decarboxylase (AADC) levels were at no time affected.[4]
It is used in scientific research in humans[5] and animals[2] to investigate the effects of serotonin depletion.
- ^ "Fenclonine" entry in Martindale – The Complete Drug Reference. Maintained in Martindale purely for archival purposes, and is no longer subject to revision and update. (Last reviewed: 2008-08-01; last modified: 2011-09-12). The Royal Pharmaceutical Society of Great Britain 2014
- ^ a b Jouvet M (August 1999). "Sleep and serotonin: an unfinished story". Neuropsychopharmacology. 21 (2 Suppl): 24S–27S. doi:10.1016/S0893-133X(99)00009-3. PMID 10432485.
- ^ Kvols LK (December 1986). "Metastatic carcinoid tumors and the carcinoid syndrome. A selective review of chemotherapy and hormonal therapy". The American Journal of Medicine. 81 (6B): 49–55. doi:10.1016/0002-9343(86)90584-x. PMID 2432781.
- ^ Park DH, Stone DM, Baker H, Kim KS, Joh TH (March 1994). "Early induction of rat brain tryptophan hydroxylase (TPH) mRNA following parachlorophenylalanine (PCPA) treatment". Brain Research. Molecular Brain Research. 22 (1–4): 20–8. doi:10.1016/0169-328x(94)90028-0. PMID 8015380.
- ^ Ruhé HG, Mason NS, Schene AH (April 2007). "Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies". Molecular Psychiatry. 12 (4): 331–59. doi:10.1038/sj.mp.4001949. PMID 17389902.