| |
| Names | |
|---|---|
| Systematic IUPAC name
iron(3+) ethanedioate (2:3) | |
| Other names
Iron(III) oxalate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.047 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
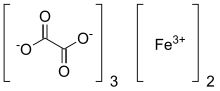
| C6Fe2O12 | |
| Molar mass | 375.747 g/mol |
| Appearance | Pale yellow solid (anhydrous) Lime green solid (hexahydrate) |
| Odor | odorless |
| Melting point | 365.1 °C (689.2 °F) |
| slightly soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe2(C2O4)3(H2O)x but could also refer to salts of [Fe(C2O4)3]3-. Fe2(C2O4)3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula [Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. This article emphasizes the coordination polymers.