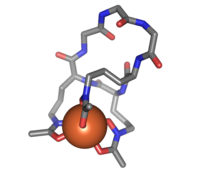
 Ferrichrome (sticks) bound to an iron atom (orange)
| |
| Names | |
|---|---|
| IUPAC name
N-[3-[4,16-bis[3-[acetyl(oxido)amino]propyl]-2,5,8,11,14,17-hexaoxo-3,6,9,12,15,18-hexazacyclooctadec-1-yl]propyl]-N-oxidoacetamide; iron(3+)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.036.081 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H42FeN9O12 | |
| Molar mass | 740.529 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrichrome is a cyclic hexa-peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination.
Ferrichrome was first isolated in 1952, and has been found to be produced by fungi of the genera Aspergillus, Ustilago, and Penicillium.[1] However, at the time there was no understanding regarding its involvement and contribution to iron transport.[2] It was not until 1957 because of Joe Neilands' work, where he first noted that Ferrichrome was able to act as an iron transport agent.
- ^ Ferrichrome Archived 2010-01-13 at the Wayback Machine, Virtual Museum of Minerals and Molecules, University of Wisconsin
- ^ Kenneth Raymond - The Human/Bacterial Arms Race for Iron, retrieved 2021-12-04