| |
| Names | |
|---|---|
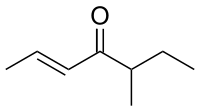
| IUPAC name
(2E)-5-Methyl-2-hepten-4-one
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.133.148 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H14O | |
| Molar mass | 126.199 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Filbertone is the principal flavor compound of hazelnuts.[1] It is used in perfumery and is designated as generally recognized as safe (GRAS) for use in food products.[2]
Because filbertone is found in hazelnut oil, its presence can be used to detect the adulteration of olive oil with less expensive hazelnut oil.[3][4]
The natural compound is a mixture of both enantiomers, and its composition can vary depending on the source.[5][6]
- ^ Jauch, Johann; Schmalzing, Dieter; Schurig, Volker; Emberger, Roland; Hopp, Rudolf; Köpsel, Manfred; Silberzahn, Wilhelm; Werkhoff, Peter (1989). "Isolation, Synthesis, and Absolute Configuration of Filbertone - the Principal Flavor Component of the Hazelnut". Angewandte Chemie International Edition in English. 28 (8): 1022. doi:10.1002/anie.198910221.
- ^ Zarbin, Paulo H.G.; Yonashiro, Massami; Perissini Jr, Waldir (1998). "An Alternative Route for the Synthesis of (E)-(+)-5(S)-Methylhept-2-en-4-one (Filbertone)". Journal of the Brazilian Chemical Society. 9 (6): 583. doi:10.1590/S0103-50531998000600011.
- ^ Ruiz Del Castillo, María Luisa; Caja, María del Mar; Herraiz, Marta; Blanch, Gracia P. (1998). "Rapid Recognition of Olive Oil Adulterated with Hazelnut Oil by Direct Analysis of the Enantiomeric Composition of Filbertone". Journal of Agricultural and Food Chemistry. 46 (12): 5128. doi:10.1021/jf9807014.
- ^ Flores, Gema; Ruiz Del Castillo, Maria Luisa; Herraiz, Marta; Blanch, Gracia Patricia (2006). "Study of the adulteration of olive oil with hazelnut oil by on-line coupled high performance liquid chromatographic and gas chromatographic analysis of filbertone". Food Chemistry. 97 (4): 742. doi:10.1016/j.foodchem.2005.06.008.
- ^ Ruiz Del Castillo, M. L.; Gómez Caballero, E.; Blanch, G. P.; Herraiz, M. (2002). "Enantiomeric composition of filbertone in hazelnuts and hazelnut oils from different geographical origins". Journal of the American Oil Chemists' Society. 79 (6): 589. doi:10.1007/s11746-002-0527-1. hdl:10261/241822. S2CID 94014818.
- ^ Güntert, Matthias; Emberger, Roland; Hopp, Rudolf; Köpsel, Manfred; Silberzahn, Wilhelm; Werkhoff, Peter (1991). "Chirospecific analysis in flavor and essential oil chemistry Part A. Filbertone ? The character impact compound of hazel-nuts". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 192 (2): 108. doi:10.1007/BF01202621. S2CID 92237130.