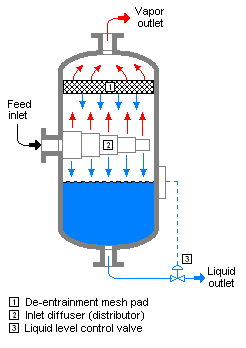
Flash evaporation (or partial evaporation) is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations. If the throttling valve or device is located at the entry into a pressure vessel so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a flash drum.[1][2]
If the saturated liquid is a single-component liquid (for example, propane or liquid ammonia), a part of the liquid immediately "flashes" into vapor. Both the vapor and the residual liquid are cooled to the saturation temperature of the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional vapor compression refrigeration systems.
If the saturated liquid is a multi-component liquid (for example, a mixture of propane, isobutane and normal butane), the flashed vapor is richer in the more volatile components than is the remaining liquid.
Uncontrolled flash evaporation can result in a boiling liquid expanding vapor explosion (BLEVE).