 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Addyi |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 33%[3] |
| Protein binding | ~98% |
| Metabolism | Extensive by liver (mainly by CYP3A4 and CYP2C19) |
| Elimination half-life | ~11 hours |
| Excretion | Bile duct (51%), kidney (44%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.970 |
| Chemical and physical data | |
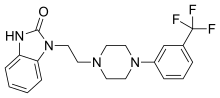
| Formula | C20H21F3N4O |
| Molar mass | 390.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Flibanserin, sold under the brand name Addyi, is a medication approved for the treatment of pre-menopausal women with hypoactive sexual desire disorder (HSDD).[4][5] The medication improves sexual desire, increases the number of satisfying sexual events, and decreases the distress associated with low sexual desire.[6] The most common side effects are dizziness, sleepiness, nausea, difficulty falling asleep or staying asleep and dry mouth.[6]
Development by Boehringer Ingelheim was halted in October 2010, following a negative evaluation by the US Food and Drug Administration (FDA).[7] The rights to the drug were then transferred to Sprout Pharmaceuticals, which achieved approval of the drug by the US FDA in August 2015.[8]
Addyi is approved for medical use in the US for premenopausal women with HSDD and in Canada for premenopausal and postmenopausal women with HSDD.[6][9]
HSDD was recognized as a distinct sexual function disorder for more than 30 years, but was removed from the Diagnostic and Statistical Manual of Mental Disorders in 2013, and replaced with a new diagnosis called female sexual interest/arousal disorder (FSIAD).[10][11]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Regulatory Decision Summary for Addyi". 23 October 2014.
- ^ a b "Addyi- flibanserin tablet, film coated". DailyMed. 10 October 2019. Retrieved 20 October 2020.
- ^ Borsini F, Evans K, Jason K, Rohde F, Alexander B, Pollentier S (2002). "Pharmacology of flibanserin". CNS Drug Reviews. 8 (2): 117–142. doi:10.1111/j.1527-3458.2002.tb00219.x. PMC 6741686. PMID 12177684.
- ^ Jolly E, Clayton A, Thorp J, Lewis-D'Agostino D, Wunderlich G, Lesko L (April 2008). "Design of Phase III pivotal trials of flibanserin in female Hypoactive Sexual Desire Disorder (HSDD)". Sexologies. 17 (Suppl 1): S133–4. doi:10.1016/S1158-1360(08)72886-X.
- ^ a b c "ADDYI- flibanserin tablet, film coated". DailyMed. 1 September 2021. Retrieved 14 November 2022.
- ^ Spiegel online: Pharmakonzern stoppt Lustpille für die Frau, 8 October 2010 (in German)
- ^ Mullard A (October 2015). "FDA approves female sexual dysfunction drug". Nature Reviews. Drug Discovery. 14 (10): 669. doi:10.1038/nrd4757. PMID 26424353. S2CID 36380932.
- ^ "ADDYI Product Monograph" (PDF). Health Canada. 26 January 2021. Retrieved 14 November 2022.
- ^ American Psychiatric Association. Sexual and gender identity disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000:493–538.
- ^ Nagoski E (27 February 2015). "Nothing Is Wrong With Your Sex Drive". The New York Times. Retrieved 31 July 2017.