| |
| Names | |
|---|---|
| Other names
Sulfurofluoridoite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
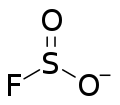
| FO2S−1 | |
| Molar mass | 83.06 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluorosulfite is an ion with the formula SO2F−. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier.[1]
Organic compounds with the name "fluorosulfite" contain the group -OS(O)F.[2]
- ^ Cite error: The named reference
:2was invoked but never defined (see the help page). - ^ Baasner, Bernd (2014). Houben-Weyl Methods of Organic Chemistry Vol. E 10a, 4th Edition Supplement: Organo-Fluorine Compounds - Fluorinating Agents and Their Application in Organic Synthesis. Georg Thieme Verlag. pp. 332–334. ISBN 978-3-13-181544-6.