 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Monuril, Monurol, Ivozfo, others |
| Other names | Phosphomycin, phosphonomycin, fosfomycin tromethamine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697008 |
| License data |
|
| Routes of administration | Intravenous, By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30–37% (by mouth, fosfomycin tromethamine); varies with food intake |
| Protein binding | Nil |
| Metabolism | Nil |
| Elimination half-life | 5.7 hours (mean) |
| Excretion | Kidney, unchanged |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.315 |
| Chemical and physical data | |
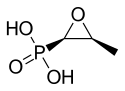
| Formula | C3H7O4P |
| Molar mass | 138.059 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 94 °C (201 °F) |
| |
| |
| (verify) | |
Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat lower urinary tract infections.[8] It is not indicated for kidney infections.[8] Occasionally it is used for prostate infections.[8] It is generally taken by mouth.[8]
Common side effects include diarrhea, nausea, headache, and vaginal yeast infections.[8] Severe side effects may include anaphylaxis and Clostridioides difficile-associated diarrhea.[8] While use during pregnancy has not been found to be harmful, such use is not recommended.[9] A single dose when breastfeeding appears safe.[9] Fosfomycin works by interfering with the production of the bacterial cell wall.[8]
Fosfomycin was discovered in 1969 and approved for medical use in the United States in 1996 [globalize][8][10] It is on the World Health Organization's List of Essential Medicines.[11] The World Health Organization classifies fosfomycin as critically important for human medicine.[12] It is available as a generic medication.[13] It was originally produced by certain types of Streptomyces, although it is now made chemically.[10]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 9 April 2023.
- ^ "Prescription medicines and biologicals: TGA annual summary 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 31 March 2024.
- ^ https://www.tga.gov.au/resources/prescription-medicines-registrations/cipfosin-cipla-fosfomycin-cipla-australia-pty-ltd
- ^ "Regulatory Decision Summary - Ivozfo". Health Canada. 23 October 2014. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ^ "Monuril 3g granules for oral solution - Summary of Product Characteristics (SmPC)". (emc). 1 June 2021. Archived from the original on 8 March 2022. Retrieved 7 June 2022.
- ^ "Fomicyt 40 mg/mL powder for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). 11 February 2021. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ^ "Monurol- fosfomycin tromethamine powder". DailyMed. 24 October 2019. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ^ a b c d e f g h "Fosfomycin Tromethamine Monograph for Professionals". Drugs.com. Archived from the original on 29 October 2019. Retrieved 29 October 2019.
- ^ a b "Fosfomycin (Monurol) Use During Pregnancy". Drugs.com. Archived from the original on 29 October 2019. Retrieved 29 October 2019.
- ^ a b Finch RG, Greenwood D, Whitley RJ, Norrby SR (2010). Antibiotic and Chemotherapy E-Book. Elsevier Health Sciences. p. 259. ISBN 9780702047657.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2019). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. hdl:10665/312266. ISBN 9789241515528.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 560–561. ISBN 9780857113382.