 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Frova |
| Other names | 6-methylamino-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide (6R)-6-methylamino-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604013 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20–30% |
| Metabolism | Hepatic |
| Elimination half-life | 26 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
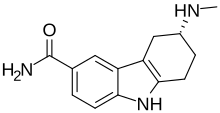
| Formula | C14H17N3O |
| Molar mass | 243.310 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Frovatriptan, sold under the brand name Frova, is a triptan drug developed by Vernalis for the treatment of migraine headaches[1] and for short term prevention of menstrual migraine.[2] The product is licensed to Endo Pharmaceuticals in North America and Menarini in Europe.[3]
- ^ Allais G, Benedetto C (2016). "Spotlight on frovatriptan: a review of its efficacy in the treatment of migraine". Drug Design, Development and Therapy. 10: 3225–3236. doi:10.2147/DDDT.S105932. PMC 5055118. PMID 27757013.
- ^ MacGregor EA (2014). "A review of frovatriptan for the treatment of menstrual migraine". International Journal of Women's Health. 6: 523–35. doi:10.2147/IJWH.S63444. PMC 4039425. PMID 24904224.
- ^ "Frova". Vernalis. Archived from the original on 2007-09-27. Retrieved 2007-11-28.