| |
| Names | |
|---|---|
| IUPAC name
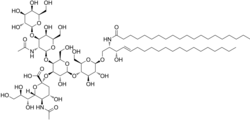
(2S,4S,5R,6R)-5-acetamido-2-[(2S,3R,4R,5S,6R)-5-[(2S,3R,4R,5R,6R)-3-acetamido-5-hydroxy-6-(hydroxymethyl)-4-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2-[(2R,3S,4R,5R,6R)-4,5-dihydroxy-6-[(E,2R,3S)-3-hydroxy-2-(icosanoylamino)icos-4-enoxy]-2-(hydroxymethyl)oxan-3-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid
| |
| Other names
Monosialotetrahexosylganglioside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | G(M1)+Ganglioside |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C77H139N3O31 | |
| Molar mass | 1602.949 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
GM1 (monosialotetrahexosylganglioside) the "prototype" ganglioside, is a member of the ganglio series of gangliosides which contain one sialic acid residue. GM1 has important physiological properties and impacts neuronal plasticity and repair mechanisms, and the release of neurotrophins in the brain. Besides its function in the physiology of the brain, GM1 acts as the site of binding for both cholera toxin and E. coli heat-labile enterotoxin (Traveller's diarrhea).[1][2]
- ^ Mocchetti I (2005). "Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins". Cell. Mol. Life Sci. 62 (19–20): 2283–94. doi:10.1007/s00018-005-5188-y. PMC 11139125. PMID 16158191.
- ^ Chen JC, Chang YS, Wu SL, et al. (September 2007). "Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa". J Ethnopharmacol. 113 (2): 233–9. doi:10.1016/j.jep.2007.05.031. PMID 17624704.