In chemistry, the descriptor geminal (from Latin gemini 'twins'[1]) refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to the same carbon atom, as in methanediol. Also the shortened prefix gem may be applied to a chemical name to denote this relationship, as in a gem-dibromide for "geminal dibromide".[citation needed]
The concept is important in many branches of chemistry, including synthesis and spectroscopy, because functional groups attached to the same atom often behave differently from when they are separated. Geminal diols, for example, are easily converted to ketones or aldehydes with loss of water.[2]
| Alkane | geminal | vicinal | isolated | |
| Methane | 
|
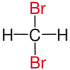
|
not existing | not existing |
| Ethane | 
|
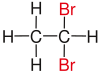
|

|
not existing |
| Propane | 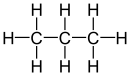
|
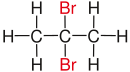
|

|
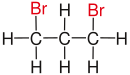
|
| Substituents on selected dibromoalkanes labeled red. | ||||
The related term vicinal refers to the relationship between two functional groups that are attached to adjacent atoms. This relative arrangement of two functional groups can also be described by the descriptors α and β.
- ^ "Definition of GEMINI". Merriam-Webster Dictionary. 27 January 2021. Retrieved 27 January 2021.
- ^ Peter Taylor (2002), Mechanism and synthesis, Book 10 of Molecular world. Open University, Royal Society of Chemistry; ISBN 0-85404-695-X. 368 pages