This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
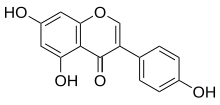
| |

| |
| Names | |
|---|---|
| IUPAC name
4′,5,7-Trihydroxyisoflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| 263823 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.524 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.[1]
It was first isolated in 1899 from the dyer's broom, Genista tinctoria; hence, the chemical name. The compound structure was established in 1926, when it was found to be identical with that of prunetol. It was chemically synthesized in 1928.[2] It has been shown to be the primary secondary metabolite of the Trifolium species and Glycine max.[3]
- ^ Sail, Vibhavari; Hadden, M. Kyle (2012-01-01), "Notch Pathway Modulators as Anticancer Chemotherapeutics", in Desai, Manoj C. (ed.), Chapter Eighteen - Notch Pathway Modulators as Anticancer Chemotherapeutics, Annual Reports in Medicinal Chemistry, vol. 47, Academic Press, pp. 267–280, doi:10.1016/B978-0-12-396492-2.00018-7, ISBN 978-0-12-396492-2, retrieved 2020-09-14
- ^ Walter, E. D. (1941). "Genistin (an Isoflavone Glucoside) and its Aglucone, Genistein, from Soybeans". Journal of the American Chemical Society. 63 (12): 3273–76. doi:10.1021/ja01857a013.
- ^ Popiołkiewicz, Joanna; Polkowski, Krzysztof; Skierski, Janusz S.; Mazurek, Aleksander P. (November 2005). "In vitro toxicity evaluation in the development of new anticancer drugs—genistein glycosides". Cancer Letters. 229 (1): 67–75. doi:10.1016/j.canlet.2005.01.014. ISSN 0304-3835. PMID 16157220.
