| |
| Names | |
|---|---|
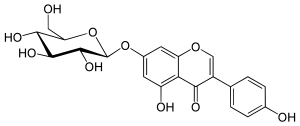
| IUPAC name
7-(β-D-Glucopyranosyloxy)-4′,5-dihydroxyisoflavone
| |
| Systematic IUPAC name
5-Hydroxy-3-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Genistoside
Genistine Genistein 7-glucoside Genistein glucoside Genistein-7-glucoside Genisteol 7-monoglucoside Glucosyl-7-genistein Genistein 7-O-beta-D-glucoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.120.406 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20O10 | |
| Molar mass | 432.37 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Genistin is an isoflavone found in a number of dietary plants like soy and kudzu. It was first isolated in 1931 from the 90% methanol extract of a soybean meal, when it was found that hydrolysis with hydrochloric acid produced 1 mole each of genistein and glucose.[1] Chemically it is the 7-O-beta-D-glucoside form of genistein and is the predominant form of the isoflavone naturally occurring in plants. In fact, studies in the 1970s revealed that 99% of the isoflavonoid compounds in soy are present as their glucosides. The glucosides are converted by digestive enzymes in the digestive system to exert their biological effects. Genistin is also converted to a more familiar genistein, thus, the biological activities including antiatherosclerotic, estrogenic and anticancer effects are analogous.
- ^ Walter ED (1941). "Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans". J Am Chem Soc. 62 (12): 3273–3276. doi:10.1021/ja01857a013.