| |
| Names | |
|---|---|
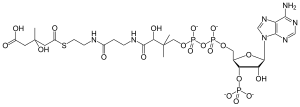
| IUPAC name
(9R,21S)-1-[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9,21-tetrahydroxy-8,8,21-trimethyl-10,14,19-trioxo-2,4,6-trioxa-18-thia-11,15-diaza-3,5-diphosphatricosan-23-oic acid 3,5-dioxide
| |
| Other names
3-hydroxy-3-methylglutaryl CoA; 3-hydroxy-3-methylglutaryl coenzyme A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.820 |
| MeSH | HMG-CoA |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H44N7O20P3S | |
| Molar mass | 911.661 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.[1][2]
HMG-CoA is a metabolic intermediate in the metabolism of the branched-chain amino acids, which include leucine, isoleucine, and valine.[3] Its immediate precursors are β-methylglutaconyl-CoA (MG-CoA) and β-hydroxy β-methylbutyryl-CoA (HMB-CoA).[4][5][6]
HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol.
- ^ Sarkar DP (2015). "Classics in Indian Medicine" (PDF). The National Medical Journal of India (28): 3. Archived from the original (PDF) on 2016-05-31.
- ^ Surolia A (1997). "An outstanding scientist and a splendid human being". Glycobiology. 7 (4): v–ix. doi:10.1093/glycob/7.4.453.
- ^ "Valine, leucine and isoleucine degradation - Reference pathway". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. 27 January 2016. Retrieved 1 February 2018.
- ^ Cite error: The named reference
ISSN position stand 2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Leucine metabolismwas invoked but never defined (see the help page).