 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fluothane |
| AHFS/Drugs.com | FDA Professional Drug Information |
| License data | |
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2E1[4]) |
| Excretion | Kidney, respiratory |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.270 |
| Chemical and physical data | |
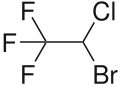
| Formula | C2HBrClF3 |
| Molar mass | 197.38 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.871 g/cm3 (at 20 °C) |
| Melting point | −118 °C (−180 °F) |
| Boiling point | 50.2 °C (122.4 °F) |
| |
| |
| (verify) | |
Halothane, sold under the brand name Fluothane among others, is a general anaesthetic.[5] It can be used to induce or maintain anaesthesia.[5] One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate.[5] It is given by inhalation.[5]
Side effects include an irregular heartbeat, respiratory depression, and hepatotoxicity.[5] Like all volatile anesthetics, it should not be used in people with a personal or family history of malignant hyperthermia.[5] It appears to be safe in porphyria.[6] It is unclear whether its usage during pregnancy is harmful to the fetus, and its use during a C-section is generally discouraged.[7] Halothane is a chiral molecule that is used as a racemic mixture.[8]
Halothane was discovered in 1951.[9] It was approved for medical use in the United States in 1958.[3] It is on the World Health Organization's List of Essential Medicines.[10] Its use in developed countries has been mostly replaced by newer anesthetic agents such as sevoflurane.[11] It is no longer commercially available in the United States.[7] Halothane also contributes to ozone depletion.[12][13]
- ^ Anvisa (31 March 2023). "RDC Nº 784 — Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 — Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Halothane, USP". DailyMed. 18 September 2013. Retrieved 11 February 2022.
- ^ a b "Fluothane: FDA-Approved Drugs". U.S. Food and Drug Administration. Retrieved 12 February 2022.
- ^ "Halothane". DrugBank. DB01159.
- ^ a b c d e f World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 17–8. hdl:10665/44053. ISBN 978-92-4-154765-9.
- ^ James MF, Hift RJ (July 2000). "Porphyrias". British Journal of Anaesthesia. 85 (1): 143–53. doi:10.1093/bja/85.1.143. PMID 10928003.
- ^ a b "Halothane — FDA prescribing information, side effects and uses". www.drugs.com. June 2005. Archived from the original on 21 December 2016. Retrieved 13 December 2016.
- ^ Bricker S (17 June 2004). The Anaesthesia Science Viva Book. Cambridge University Press. p. 161. ISBN 978-0-521-68248-0. Archived from the original on 10 September 2017 – via Google Books.
- ^ Walker SR (2012). Trends and Changes in Drug Research and Development. Springer. p. 109. ISBN 978-94-009-2659-2. Archived from the original on 10 September 2017.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Yentis SM, Hirsch NP, Ip J (2013). Anaesthesia and Intensive Care A-Z: An Encyclopedia of Principles and Practice (5th ed.). Elsevier Health Sciences. p. 264. ISBN 978-0-7020-5375-7. Archived from the original on 10 September 2017.
- ^ Kümmerer K (2013). Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks. Springer. p. 33. ISBN 978-3-662-09259-0.
- ^ Langbein T, Sonntag H, Trapp D, Hoffmann A, Malms W, Röth EP, et al. (January 1999). "Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane". British Journal of Anaesthesia. 82 (1): 66–73. doi:10.1093/bja/82.1.66. PMID 10325839.