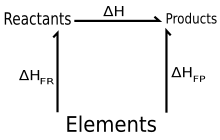
Hess’ law of constant heat summation, also known simply as Hess' law, is a relationship in physical chemistry named after Germain Hess, a Swiss-born Russian chemist and physician who published it in 1840. The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken.[1][2]
Hess' law is now understood as an expression of the fact that the enthalpy of a chemical process is independent of the path taken from the initial to the final state (i.e. enthalpy is a state function). According to the first law of thermodynamics, the enthalpy change in a system due to a reaction at constant pressure is equal to the heat absorbed (or the negative of the heat released), which can be determined by calorimetry for many reactions. The values are usually stated for reactions with the same initial and final temperatures and pressures (while conditions are allowed to vary during the course of the reactions). Hess' law can be used to determine the overall energy required for a chemical reaction that can be divided into synthetic steps that are individually easier to characterize. This affords the compilation of standard enthalpies of formation, which may be used to predict the enthalpy change in complex synthesis.
- ^ Mannam Krishnamurthy; Subba Rao Naidu (2012). "7". In Lokeswara Gupta (ed.). Chemistry for ISEET - Volume 1, Part A (2012 ed.). Hyderabad, India: Varsity Education Management Limited. p. 244.
- ^ "Hess' Law - Conservation of Energy". University of Waterloo. Archived from the original on 9 January 2015. Retrieved 12 January 2014.