| |
| Names | |
|---|---|
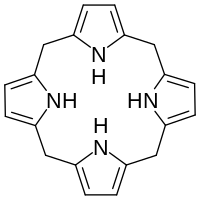
| IUPAC name
5,10,15,20,22,24-Hexahydroporphyrin
| |
| Other names
Porphyrinogen; Calix[4]pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 7545460 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H20N4 | |
| Molar mass | 316.408 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 185 °C (365 °F; 458 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexahydroporphine is an organic chemical compound with formula C20H20N4. The molecule consists of four pyrrole rings connected by methylene bridges −CH2− into a larger (non-aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one. As indicated by the name, it may be viewed as derived from porphine by the addition of six hydrogen atoms: four on the methine bridges, and two on the nitrogen atoms.
Hexahydroporphine does not occur in nature, but is the core of porphyrinogens such as uroporphyrinogen III (UROGEN), which are precursors of many porphyrins — derivatives of porphine of great biological importance. The six hydrogens of that core are removed at a later metabolic stage by the enzyme protoporphyrinogen oxidase. Because of this connection, the compound is also called (unsubstituted) porphyrinogen.
The compound is a colorless solid, soluble in dichloromethane, acetone, and diethyl ether. It decomposes at 185°C.[1]