
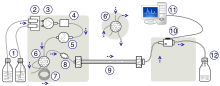
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals,[1] biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.[citation needed]
It relies on high pressure pumps, which deliver mixtures of various solvents, called the mobile phase, which flows through the system, collecting the sample mixture on the way, delivering it into a cylinder, called the column, filled with solid particles, made of adsorbent material, called the stationary phase.[2]
Each component in the sample interacts differently with the adsorbent material, causing different migration rates for each component.[3] These different rates lead to separation as the species flow out of the column into a specific detector such as UV detectors. The output of the detector is a graph, called a chromatogram. Chromatograms are graphical representations of the signal intensity versus time or volume, showing peaks, which represent components of the sample. Each sample appears in its respective time, called its retention time, having area proportional to its amount.[2]
HPLC is widely used for manufacturing (e.g., during the production process of pharmaceutical and biological products),[4][5] legal (e.g., detecting performance enhancement drugs in urine),[6] research (e.g., separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (e.g., detecting vitamin D levels in blood serum) purposes.[7]
Chromatography can be described as a mass transfer process involving adsorption and/or partition. As mentioned, HPLC relies on pumps to pass a pressurized liquid and a sample mixture through a column filled with adsorbent, leading to the separation of the sample components. The active component of the column, the adsorbent, is typically a granular material made of solid particles (e.g., silica, polymers, etc.), 1.5–50 μm in size, on which various reagents can be bonded.[8][9] The components of the sample mixture are separated from each other due to their different degrees of interaction with the adsorbent particles. The pressurized liquid is typically a mixture of solvents (e.g., water, buffers, acetonitrile and/or methanol) and is referred to as a "mobile phase". Its composition and temperature play a major role in the separation process by influencing the interactions taking place between sample components and adsorbent.[10] These interactions are physical in nature, such as hydrophobic (dispersive), dipole–dipole and ionic, most often a combination.[11][12]
- ^ Kazakevich, Yuri; LoBrutto, Rosario, eds. (2007). HPLC for pharmaceutical scientists. Hoboken, NJ: Wiley-Interscience. ISBN 978-0-471-68162-5.
- ^ a b "Chromatography". web.njit.edu. Retrieved 2024-08-05.
- ^ Chaitali Dattatray Harde1, Dr. Amol Navnath Khedkar, Vaishnavi Sanjay Sake. "Review on High Performance Liquid Chromatography" (PDF).
{{cite web}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Levin, Shulamit (January 2004). "Reversed Phase Stationary Phases in Pharmaceutical Sciences". Journal of Liquid Chromatography & Related Technologies. 27 (7–9): 1353–1376. doi:10.1081/JLC-120030606. ISSN 1082-6076. S2CID 97490509.
- ^ Gerber, F.; Krummen, M.; Potgeter, H.; Roth, A.; Siffrin, C.; Spoendlin, C. (2004). "Practical aspects of fast reversed-phase high-performance liquid chromatography using 3μm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice". Journal of Chromatography A. 1036 (2): 127–133. doi:10.1016/j.chroma.2004.02.056. PMID 15146913.
- ^ Bayne, Shirley; Carlin, Michelle (2017). Forensic Applications of High Performance Liquid Chromatography (1st ed.). CRC Press. ISBN 9780429251962.
- ^ Seger, Christoph; Salzmann, Linda (2020-08-01). "After another decade: LC–MS/MS became routine in clinical diagnostics". Clinical Biochemistry. Advancement and Applications of Mass Spectrometry in Laboratory Medicine. 82: 2–11. doi:10.1016/j.clinbiochem.2020.03.004. ISSN 0009-9120. PMID 32188572. S2CID 213186669.
- ^ Unger, K. K., ed. (1979-01-01), Chapter 5 Silica columns–packing procedures and performance characteristics, Journal of Chromatography Library, vol. 16, Elsevier, pp. 169–186, doi:10.1016/S0301-4770(08)60809-X, ISBN 978-0-444-41683-4, retrieved 2024-08-05
- ^ Xu, Yan; Cao, Qing; Svec, Frantisek; Fréchet, Jean M.J. (2010-04-15). "Porous polymer monolithic column with surface-bound gold nanoparticles for the capture and separation of cysteine-containing peptides". Analytical Chemistry. 82 (8): 3352–3358. doi:10.1021/ac1002646. ISSN 0003-2700. PMC 2875083. PMID 20302345.
- ^ Panella, Cristina; Ferretti, Rosella; Casulli, Adriano; Cirilli, Roberto (2019-10-01). "Temperature and eluent composition effects on enantiomer separation of carvedilol by high-performance liquid chromatography on immobilized amylose-based chiral stationary phases". Journal of Pharmaceutical Analysis. 9 (5): 324–331. doi:10.1016/j.jpha.2019.04.002. ISSN 2095-1779. PMC 6951491. PMID 31929941.
- ^ "Molecular Interaction of HPLC Stationary Phase". www.imtakt.com. Retrieved 2024-08-08.
- ^ Kadlecová, Zuzana; Kalíková, Květa; Folprechtová, Denisa; Tesařová, Eva; Gilar, Martin (2020-08-16). "Method for evaluation of ionic interactions in liquid chromatography". Journal of Chromatography A. 1625: 461301. doi:10.1016/j.chroma.2020.461301. ISSN 0021-9673. PMID 32709344.