| |
| |
| Names | |
|---|---|
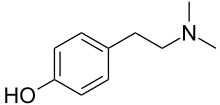
| Preferred IUPAC name
4-[2-(Dimethylamino)ethyl]phenol | |
| Other names
N,N-Dimethyltyramine; Peyocactin; Anhaline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.920 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H15NO | |
| Molar mass | 165.236 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 116 to 117 °C (241 to 243 °F; 389 to 390 K) |
| Boiling point | 173 °C (343 °F; 446 K) at 11 mm Hg; sublimes at 140–150 °C |
| high in: ethanol; ether; chloroform | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley (Hordeum species). Chemically, hordenine is the N-methyl derivative of N-methyltyramine, and the N,N-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties (see below). As of September 2012[update], hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism. In experimental animals, given sufficiently large doses parenterally (by injection), hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems. These effects are generally not reproduced by oral administration of the drug in test animals, and virtually no scientific reports of the effects of hordenine in human beings have been published.