| |
 | |
| Names | |
|---|---|
| Other names
Aminosulfuric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.065 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H3NO4S | |
| Molar mass | 113.09 |
| Appearance | white solid |
| Melting point | 210 °C |
| cold water | |
| Acidity (pKa) | 1.48[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
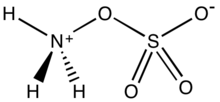
Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum.[2] It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion[3] and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.[3][4][5]
- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 115. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ Cite error: The named reference
LabPrepwas invoked but never defined (see the help page). - ^ a b Wiberg, Egon; Wiberg, Nils (2001). "Sulfur Compounds of Nitrogen". Inorganic Chemistry. Academic Press. pp. 675–677. ISBN 978-0-12-352651-9.
- ^ Wallace, Raymond G. (1980). "Hydroxylamine-O-sulfonic acid – a versatile synthetic reagent". Aldrichimica Acta. 13 (1): 3–11.
- ^ Rademacher, P. (2014). "Product Class 7: Hydrazines and Hydrazinium Salts (40.7.1.1.9.2 – Using Hydroxylamine-O-sulfonic Acids". In Enders, Dieter; Schaumann, E. (eds.). Compounds with One Saturated Carbon–Heteroatom Bond: Amine N-Oxides, Haloamines, Hydroxylamines and Sulfur Analogues, and Hydrazines. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Vol. 40b. Georg Thieme Verlag. p. 1171. ISBN 978-3-13-172181-5.