| |
| |
| Names | |
|---|---|
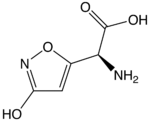
| IUPAC name
(S)-2-Amino-2-(3-hydroxyisoxazol-5-yl)acetic acid
| |
| Other names
Ibotenic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.151.170 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H6N2O4 | |
| Molar mass | 158.113 g·mol−1 |
| Melting point | 151-152 °C (anhydrous) 144-146 °C (monohydrate) |
| H2O: 1 mg/mL 0.1 M NaOH: 10.7 mg/mL 0.1 M HCl: 4.7 mg/mL | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Danger | |
| H301 | |
| P264, P270, P301+P316, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ibotenic acid or (S)-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a chemical compound and psychoactive drug which occurs naturally in Amanita muscaria and related species of mushrooms typically found in the temperate and boreal regions of the northern hemisphere. It is a prodrug of muscimol, broken down by the liver to that much more stable compound.[2] It is a conformationally-restricted analogue of the neurotransmitter glutamate, and due to its structural similarity to this neurotransmitter, acts as a non-selective glutamate receptor agonist.[3] Because of this, ibotenic acid can be a powerful neurotoxin in high doses, and is employed as a "brain-lesioning agent" through cranial injections in scientific research.[4][5] The neurotoxic effects appear to be dose-related and risks are unclear through consumption of ibotenic-acid containing fungi, although thought to be negligible in small doses.[6][better source needed]
- ^ "Ibotenic acid". pubchem.ncbi.nlm.nih.gov.
- ^ Stebelska, Katarzyna (August 2013). "Fungal Hallucinogens Psilocin, Ibotenic Acid, and Muscimol: Analytical Methods and Biologic Activities". Therapeutic Drug Monitoring. 35 (4): 420–442. doi:10.1097/FTD.0b013e31828741a5. ISSN 0163-4356. PMID 23851905. S2CID 44494685.
- ^ Tommy Liljefors; Povl Krogsgaard-Larsen; Ulf Madsen (25 July 2002). Textbook of Drug Design and Discovery, Third Edition. CRC Press. pp. 263–. ISBN 978-0-415-28288-8.
- ^ Becker, A; Grecksch, G; Bernstein, HG; Höllt, V; Bogerts, B (1999). "Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis". Psychopharmacology. 144 (4): 333–8. doi:10.1007/s002130051015. PMID 10435405. S2CID 25172395.
- ^ Isacson, O; Brundin, P; Kelly, PA; Gage, FH; Björklund, A (1984). "Functional neuronal replacement by grafted striatal neurones in the ibotenic acid-lesioned rat striatum". Nature. 311 (5985): 458–60. Bibcode:1984Natur.311..458I. doi:10.1038/311458a0. PMID 6482962. S2CID 4342937.
- ^ Filer, Crist N. (1 December 2018). "Ibotenic acid: on the mechanism of its conversion to [3H] muscimol". Journal of Radioanalytical and Nuclear Chemistry. 318 (3): 2033–2038. doi:10.1007/s10967-018-6203-8. ISSN 1588-2780. S2CID 91380050.