 | |
| Clinical data | |
|---|---|
| Trade names | Firazyr |
| Other names | Hoe 140, JE 049[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
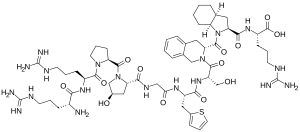
| Formula | C59H89N19O13S |
| Molar mass | 1304.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Icatibant, sold under the brand name Firazyr, is a medication for the symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults with C1-esterase-inhibitor deficiency.[5][3][4] It is not effective in angioedema caused by medication from the ACE inhibitor class.[6]
It is a peptidomimetic consisting of ten amino acids, which is a selective and specific antagonist of bradykinin B2 receptors.
- ^ "Icatibant: HOE 140, JE 049, JE049". Drugs in R&D. 5 (6): 343–8. 2004. doi:10.2165/00126839-200405060-00006. PMID 15563238. S2CID 25491021.
- ^ https://www.tga.gov.au/resources/prescription-medicines-registrations/icatibant-wkt-wockhardt-bio-pty-ltd
- ^ a b "Firazyr- icatibant acetate injection, solution". DailyMed. 16 December 2019. Retrieved 17 April 2020.
- ^ a b "Firazyr EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 17 April 2020.
- ^ "Jerini Receives European Commission Approval for Firazyr (Icatibant) in the Treatment of HAE" (Press release). Jerini AG. 15 July 2008. Retrieved 22 July 2008.[permanent dead link]
- ^ Sinert R, Levy P, Bernstein JA, Body R, Sivilotti ML, Moellman J, et al. (September–October 2017). "Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema". The Journal of Allergy and Clinical Immunology. In Practice. 5 (5): 1402–1409.e3. doi:10.1016/j.jaip.2017.03.003. PMID 28552382.
{{cite journal}}: CS1 maint: overridden setting (link)