 | |
| Clinical data | |
|---|---|
| Trade names | Catena, Raxone, Sovrima |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <1% (high first pass effect) |
| Protein binding | >99% |
| Elimination half-life | 18 hours |
| Excretion | Urine (80%) and feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
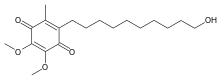
| Formula | C19H30O5 |
| Molar mass | 338.444 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Idebenone, sold under the brand name Raxone among others, is a medication that was initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer's disease and other cognitive defects.[2] This has been met with limited success. The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of neuromuscular diseases. In 2010, early clinical trials for the treatment of Friedreich's ataxia[3] and Duchenne muscular dystrophy[4] have been completed. As of December 2013[update] the drug is not approved for these indications in North America or Europe. It is approved by the European Medicines Agency (EMA) for use in Leber's hereditary optic neuropathy (LHON) and was designated an orphan drug in 2007.[5]
Chemically, idebenone is an organic compound of the quinone family. It is also promoted commercially as a synthetic analog of coenzyme Q10 (CoQ10).
- ^ "Raxone EPAR". European Medicines Agency (EMA). 15 February 2007. Retrieved 26 August 2024.
- ^ CHMP Assessment Report for Sovrima (PDF) (Report). European Medicines Agency. 20 November 2008. pp. 6, 9–11, 67f. Archived from the original (PDF) on 20 June 2018. Retrieved 30 December 2013.
- ^ Clinical trial number NCT00229632 for "Idebenone to Treat Friedreich's Ataxia" at ClinicalTrials.gov
- ^ Clinical trial number NCT00654784 for "Efficacy and Tolerability of Idebenone in Boys With Cardiac Dysfunction Associated With Duchenne Muscular Dystrophy (DELPHI)" at ClinicalTrials.gov
- ^ "Raxone". www.ema.europa.eu. 17 September 2018. Retrieved 12 July 2019.