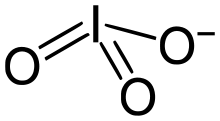
 The iodate anion, IO−3
| |
 Space-filling model of the iodate anion
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| 1676 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| IO3− | |
| Molar mass | 174.902 g·mol−1 |
| Related compounds | |
Related compounds
|
Periodate, Fluoroiodate, Bromate, Chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
An iodate is the polyatomic anion with the formula IO−3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores.[1] Iodate salts are often colorless. They are the salts of iodic acid.
- ^ Lyday, Phyllis A. (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 382–390. doi:10.1002/14356007.a14_381. ISBN 978-3527306732.