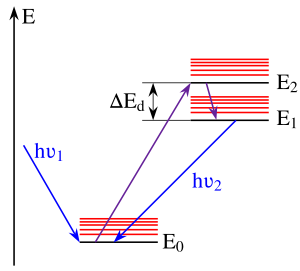
Kasha's rule is a principle in the photochemistry of electronically excited molecules. The rule states that photon emission (fluorescence or phosphorescence) occurs in appreciable yield only from the lowest excited state of a given multiplicity. It is named after American spectroscopist Michael Kasha, who proposed it in 1950.[1][2]
- ^ Characterization of Electronic Transitions in Complex Molecules. Kasha, M. Discussions of the Faraday Society, 1950, 9: p.14-19.
- ^ IUPAC. Kasha rule – Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by McNaught, A.D. and Wilkinson, A. Blackwell Scientific Publications, Oxford, 1997.





