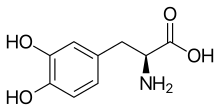
 Skeletal formula of L-DOPA
| |
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid
| |
| Other names
l-3,4-Dihydroxyphenylalanine; Levodopa
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.405 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H11NO4 | |
| Molar mass | 197.19 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants[2] and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine.
l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS.[3][4] In some plant families (of the order Caryophyllales), l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains.[5]
l-DOPA can be manufactured and in its pure form is sold as a drug with the INN levodopa. Trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
l-DOPA has a counterpart with opposite chirality, d-DOPA. As is true for many molecules, the human body produces only one of these isomers (the l-DOPA form). The enantiomeric purity of l-DOPA may be analyzed by determination of the optical rotation or by chiral thin-layer chromatography.[6]
- ^ Howard ST, Hursthouse MB, Lehmann CW, Poyner EA (1995). "Experimental and theoretical determination of electronic properties in Ldopa". Acta Crystallogr. B. 51 (3): 328–337. Bibcode:1995AcCrB..51..328H. doi:10.1107/S0108768194011407. S2CID 96802274.
- ^ Cohen PA, Avula B, Katragunta K, Khan I (October 2022). "Levodopa Content of Mucuna pruriens Supplements in the NIH Dietary Supplement Label Database". JAMA Neurology. 79 (10): 1085–1086. doi:10.1001/jamaneurol.2022.2184. PMC 9361182. PMID 35939305.
- ^ Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS (September 2008). "L-DOPA is an endogenous ligand for OA1". PLOS Biology. 6 (9): e236. doi:10.1371/journal.pbio.0060236. PMC 2553842. PMID 18828673.
- ^ Hiroshima Y, Miyamoto H, Nakamura F, Masukawa D, Yamamoto T, Muraoka H, et al. (January 2014). "The protein Ocular albinism 1 is the orphan GPCR GPR143 and mediates depressor and bradycardic responses to DOPA in the nucleus tractus solitarii". British Journal of Pharmacology. 171 (2): 403–14. doi:10.1111/bph.12459. PMC 3904260. PMID 24117106.
- ^ Polturak G, Breitel D, Grossman N, Sarrion-Perdigones A, Weithorn E, Pliner M, et al. (2016). "Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants". New Phytol. 210 (1): 269–283. doi:10.1111/nph.13796. PMID 26683006.
- ^ Martens J, Günther K, Schickedanz M (1986). "Resolution of Optical Isomers by Thin-Layer Chromatography: Enantiomeric Purity of Methyldopa". Arch. Pharm. 319 (6): 572–574. doi:10.1002/ardp.19863190618. S2CID 97903386.