| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.210 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1469 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Pb(NO3)2 | |||
| Molar mass | 331.2 g/mol | ||
| Appearance | colorless or white | ||
| Density | 4.53 g/cm3 | ||
| Melting point | 470 °C (878 °F; 743 K)[2] decomposes | ||
| 376.5 g/L (0 °C) 597 g/L (25°C) 1270 g/L (100°C) | |||
| −74·10−6 cm3/mol[1] | |||
Refractive index (nD)
|
1.782[2] | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−451.9 kJ·mol−1[1] | ||
| Hazards | |||
| GHS labelling:[4] | |||
   
| |||
| Danger | |||
| H302, H317, H318, H332, H360, H373, H410 | |||
| P201, P202, P210, P220, P221, P260, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P310, P312, P314, P321, P330, P333+P313, P363, P370+P378, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published)
|
500 mg/kg (guinea pig, oral)[3] | ||
| Safety data sheet (SDS) | ICSC 1000 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
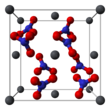
Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumbum dulce, the production of lead(II) nitrate from either metallic lead or lead oxide in nitric acid was small-scale, for direct use in making other lead compounds. In the nineteenth century lead(II) nitrate began to be produced commercially in Europe and the United States. Historically, the main use was as a raw material in the production of pigments for lead paints, but such paints have been superseded by less toxic paints based on titanium dioxide. Other industrial uses included heat stabilization in nylon and polyesters, and in coatings of photothermographic paper. Since around the year 2000, lead(II) nitrate has begun to be used in gold cyanidation.
Lead(II) nitrate is toxic and must be handled with care to prevent inhalation, ingestion and skin contact. Due to its hazardous nature, the limited applications of lead(II) nitrate are under constant scrutiny.
- ^ a b CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ a b Patnaik, Pradyot (2003). Handbook of inorganic chemicals. New York: McGraw-Hill. p. 475. ISBN 0-07-049439-8. OCLC 50252041.
- ^ "Lead compounds (as Pb)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Lead nitrate". pubchem.ncbi.nlm.nih.gov. Retrieved 19 December 2021.