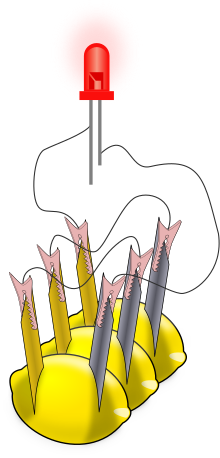
A lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and a piece of copper (such as a penny) are inserted into a lemon and connected by wires. Power generated by reaction of the metals is used to power a small device such as a light-emitting diode (LED).
The lemon battery is similar to the first electrical battery invented in 1800 by Alessandro Volta, who used brine (salt water) instead of lemon juice.[1] The lemon battery illustrates the type of chemical reaction (oxidation-reduction) that occurs in batteries.[2][3][4] The zinc and copper are called the electrodes, and the juice inside the lemon is called the electrolyte. There are many variations of the lemon cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as electrodes.
- ^ Cite error: The named reference
Deckerwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Snyderwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Oonwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Goodismanwas invoked but never defined (see the help page).