| |
| Names | |
|---|---|
| IUPAC name
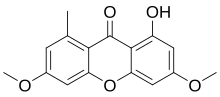
1-Hydroxy-3,6-dimethoxy-8-methyl-9H-xanthen-9-one
| |
| Other names
Lichenxanthone,
1-hydroxy-3,6-dimethoxy-8-methylxanthen-9-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H14O5 | |
| Molar mass | 286.283 g·mol−1 |
| Appearance | long yellow prismatic crystals |
| Density | 1.323 g/cm3 |
| Melting point | 189–190 °C (372–374 °F; 462–463 K) |
| Boiling point | 494 °C (921 °F) |
| Structure[1] | |
| Monoclinic | |
| P21/c (No. 14) | |
a = 11.6405 Å, b = 7.5444 Å, c = 15.2341 Å
| |
Lattice volume (V)
|
1307.26 Å3 |
Formula units (Z)
|
4 |
| Hazards | |
| Flash point | 186.9 °C (368.4 °F) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants (many are from the families Annonaceae and Rutaceae), and some species of fungi that do not form lichens.
In lichens, the biosynthesis of lichexanthone occurs through a set of enzymatic reactions that start with the molecule acetyl-CoA and sequentially add successive units, forming a longer chain that is cyclized into a double-ring structure. Although it has been suggested that lichexanthone functions in nature as a photoprotectant—protecting resident algal populations (photobionts) in lichens from high-intensity solar radiation—its complete ecological function is not fully understood. Some biological activities of lichexanthone that have been demonstrated in the laboratory include antibacterial, larvicidal, and sperm motility-enhancing activities. Many lichexanthone derivatives are known, some produced naturally in lichens, and others created synthetically; like lichexanthone, some of these derivatives are also biologically active.