 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /loʊˈpɛrəmaɪd/ |
| Trade names | Imodium, others[1] |
| Other names | R-18553, Loperamide hydrochloride (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682280 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.3% |
| Protein binding | 97% |
| Metabolism | Liver (extensive) |
| Elimination half-life | 9–14 hours[4] |
| Excretion | Feces (30–40%), urine (1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.088 |
| Chemical and physical data | |
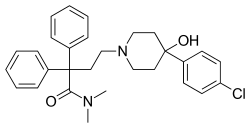
| Formula | C29H33ClN2O2 |
| Molar mass | 477.05 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Loperamide, sold under the brand name Imodium, among others,[1] is a medication of the opioid receptor agonist class used to decrease the frequency of diarrhea.[5][4] It is often used for this purpose in irritable bowel syndrome, inflammatory bowel disease, short bowel syndrome[4] Crohn's disease and ulcerative colitis.[5] It is not recommended for those with blood in the stool, mucus in the stool, or fevers.[4] The medication is taken by mouth.[4]
Common side effects include abdominal pain, constipation, sleepiness, vomiting, and a dry mouth.[4] It may increase the risk of toxic megacolon.[4] Loperamide's safety in pregnancy is unclear, but no evidence of harm has been found.[6] It appears to be safe in breastfeeding.[7] It is an opioid with no significant absorption from the gut and does not cross the blood–brain barrier when used at normal doses.[8] It works by slowing the contractions of the intestines.[4]
Loperamide was first made in 1969 and used medically in 1976.[9] It is on the World Health Organization's List of Essential Medicines.[10] Loperamide is available as a generic medication.[4][11] In 2021, it was the 287th most commonly prescribed medication in the United States, with more than 700,000 prescriptions.[12][13]
- ^ a b Drugs.com International brands for loperamide Archived 23 September 2015 at the Wayback Machine Page accessed 4 September 2015
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Loperamide hydrochloride capsule". DailyMed. 30 September 2022. Archived from the original on 14 September 2023. Retrieved 8 January 2024.
- ^ a b c d e f g h i "Loperamide Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 7 September 2015. Retrieved 25 August 2015.
- ^ a b "About loperamide". nhs.uk. 11 April 2024.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ^ "Loperamide use while Breastfeeding". Archived from the original on 8 September 2015. Retrieved 26 August 2015.
- ^ "loperamide hydrochloride". NCI Drug Dictionary. 2 February 2011. Archived from the original on 7 September 2015. Retrieved 26 August 2015.
- ^ Patrick GL (2013). An introduction to medicinal chemistry (Fifth ed.). Oxford: Oxford University Press. p. 644. ISBN 9780199697397. Archived from the original on 14 January 2023. Retrieved 17 December 2020.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton RJ (2013). Tarascon pocket pharmacopoeia (14 ed.). [Sudbury, Mass.]: Jones & Bartlett Learning. p. 217. ISBN 9781449673611. Archived from the original on 5 March 2016.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Loperamide - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.