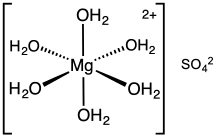
 Magnesium sulfate hexahydrate
| |
 Anhydrous magnesium sulfate
| |
 Epsomite (Magnesium sulfate heptahydrate)
| |
| Names | |
|---|---|
| IUPAC name
Magnesium sulfate
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.453 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MgSO4 | |
| Molar mass |
|
| Appearance | white crystalline solid |
| Odor | odorless |
| Density |
|
| Melting point |
|
| |
Solubility product (Ksp)
|
738 (502 g/L) |
| Solubility | |
| −50·10−6 cm3/mol | |
Refractive index (nD)
|
1.523 (monohydrate) 1.433 (heptahydrate) |
| Structure | |
| monoclinic (hydrate) | |
| Pharmacology | |
| A06AD04 (WHO) A12CC02 (WHO) B05XA05 (WHO) D11AX05 (WHO) V04CC02 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.
Magnesium sulfate is usually encountered in the form of a hydrate MgSO4·nH2O, for various values of n between 1 and 11. The most common is the heptahydrate MgSO4·7H2O,[1] known as Epsom salt, which is a household chemical with many traditional uses, including bath salts.[2]
The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year.[3] The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs.
- ^ Connor, Nick (24 July 2023). "Magnesium Sulfate | Formula, Properties & Application". Material Properties. Retrieved 4 February 2024.
- ^ Cite error: The named reference
wsjdXXXXwas invoked but never defined (see the help page). - ^ Cite error: The named reference
buch2000was invoked but never defined (see the help page).
