| |
| Names | |
|---|---|
| Other names
dimanganese trioxide, manganese sesquioxide, manganic oxide, manganous oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.878 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mn2O3 | |
| Molar mass | 157.8743 g/mol |
| Appearance | brown or black crystalline |
| Density | 4.50 g/cm3 |
| Melting point | 888 °C (1,630 °F; 1,161 K) (alpha form) 940 °C, decomposes (beta form) |
| 0.00504 g/100 mL (alpha form) 0.01065 g/100 mL (beta form) | |
| Solubility | insoluble in ethanol, acetone soluble in acid, ammonium chloride |
| +14,100·10−6 cm3/mol | |
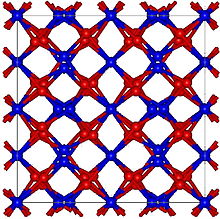
| Structure[1] | |
| Bixbyite, cI80 | |
| Ia3 (No. 206) | |
a = 942 pm
| |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
110 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−971 kJ·mol−1[2] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
manganese trifluoride, manganese(III) acetate |
Other cations
|
chromium(III) oxide, iron(III) oxide |
Related compounds
|
manganese(II) oxide, manganese dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese(III) oxide is a chemical compound with the formula Mn2O3. It occurs in nature as the mineral bixbyite (recently changed to bixbyite-(Mn)[3][4]) and is used in the production of ferrites and thermistors.
- ^ Chandiran, Kalaiselvi; Murugesan, Ramesh Aravind; Balaji, Revathi; Andrews, Nirmala Grace; Pitchaimuthu, Sudhagar; Nagamuthu Raja, Krishna Chandar (2020-07-03). "Long single crystalline α-Mn2O3 nanorods: facile synthesis and photocatalytic application". Materials Research Express. 7 (7). IOP Publishing: 074001. doi:10.1088/2053-1591/ab9fbd. ISSN 2053-1591. S2CID 225561660.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Bixbyite-(Mn)".
- ^ IMA 21-H: Redefinition of bixbyite and definition of bixbyite-(Fe) and bixbyite-(Mn). CNMNC Newsletter, 64, 2021; Mineralogical Magazine, 85, 2021).
