 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Osmitrol, Bronchitol, others |
| Other names | d-Mannitol, mannite, manna sugar |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~7% |
| Metabolism | Liver, negligible |
| Elimination half-life | 100 minutes |
| Excretion | Kidney: 90% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | E421 (thickeners, ...) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.647 |
| Chemical and physical data | |
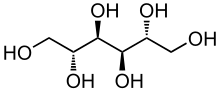
| Formula | C6H14O6 |
| Molar mass | 182.172 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mannitol is a type of sugar alcohol used as a sweetener and medication.[5][6] It is used as a low calorie sweetener as it is poorly absorbed by the intestines.[5] As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower increased intracranial pressure.[7][8][6] Medically, it is given by injection or inhalation.[9][10] Effects typically begin within 15 minutes and last up to 8 hours.[9]
Common side effects from medical use include electrolyte problems and dehydration.[9] Other serious side effects may include worsening heart failure and kidney problems.[9][6] It is unclear if use is safe in pregnancy.[9] Mannitol is in the osmotic diuretic family of medications and works by pulling fluid from the brain and eyes.[9]
The discovery of mannitol is attributed to Joseph Louis Proust in 1806.[11] It is on the World Health Organization's List of Essential Medicines.[12] It was originally made from the flowering ash and called manna due to its supposed resemblance to the Biblical food.[13][14] Mannitol is on the World Anti-Doping Agency's banned drug list due to concerns that it may mask other drugs.[15]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Regulatory Decision Summary - Aridol". Health Canada. 23 October 2014. Retrieved 7 June 2022.
- ^ "Osmitrol- mannitol injection, solution". DailyMed. 15 November 2018. Retrieved 28 October 2020.
- ^ "Bronchitol EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 28 October 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b Varzakas T, Labropoulos A, Anestis S (2012). Sweeteners: Nutritional Aspects, Applications, and Production Technology. CRC Press. pp. 59–60. ISBN 9781439876732. Archived from the original on 10 September 2017.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 332. hdl:10665/44053. ISBN 9789241547659.
- ^ Wakai A, McCabe A, Roberts I, Schierhout G (August 2013). "Mannitol for acute traumatic brain injury". The Cochrane Database of Systematic Reviews. 2013 (8): CD001049. doi:10.1002/14651858.CD001049.pub5. PMC 7050611. PMID 23918314.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 332. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d e f "Mannitol". The American Society of Health-System Pharmacists. Archived from the original on 26 May 2015. Retrieved 8 January 2015.
- ^ "BRONCHITOL® (mannitol) inhalation powder Patient Site". bronchitol.com.
- ^ Kremers E, Sonnedecker G (1986). Kremers and Urdang's History of Pharmacy. Amer. Inst. History of Pharmacy. p. 360. ISBN 9780931292170. Archived from the original on 10 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Cottrell JE, Patel P (2016). Cottrell and Patel's Neuroanesthesia. Elsevier Health Sciences. p. 160. ISBN 9780323461122.
- ^ Bardal S, Waechter J, Martin D (2010). Applied Pharmacology. Elsevier Health Sciences. p. 411. ISBN 978-1437735789.
- ^ "THE 2017 PROHIBITED LIST INTERNATIONAL STANDARD" (PDF). January 2017. p. 5. Retrieved 7 July 2018.