 | |
| Clinical data | |
|---|---|
| Routes of administration | Topical (ointment, eye drops) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.969 |
| Chemical and physical data | |
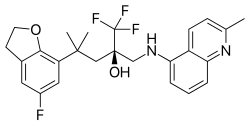
| Formula | C25H26F4N2O2 |
| Molar mass | 462.489 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mapracorat (INN, code names BOL-303242-X, ZK-245186[1]) is an anti-inflammatory drug belonging to the experimental class of selective glucocorticoid receptor agonists (SEGRAs). It is in clinical trials for the topical treatment of atopic dermatitis,[2] inflammation following cataract surgery,[3] and allergic conjunctivitis.[4] Preliminary investigation for the treatment of keratoconjunctivitis sicca has been conducted in cellular models.[1]
- ^ a b Cavet ME, Harrington KL, Ward KW, Zhang JZ (September 2010). "Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells". Molecular Vision. 16: 1791–800. PMC 2932489. PMID 20824100.
- ^ Clinical trial number NCT00944632 for "Dose Escalation of Different Concentrations of ZK 245186 in Atopic Dermatitis" at ClinicalTrials.gov
- ^ Clinical trial number NCT00905450 for "Evaluation of BOL-303242-X Versus Vehicle for the Treatment of Inflammation Following Cataract Surgery" at ClinicalTrials.gov
- ^ Clinical trial number NCT01289431 for "Mapracorat Ophthalmic Formulation in Subjects With Allergic Conjunctivitis" at ClinicalTrials.gov