 | |
| Clinical data | |
|---|---|
| Trade names | Bonine, Antivert, others |
| Other names | Meclozine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682548 |
| License data | |
| Routes of administration | By mouth, under the tongue, in the cheek |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 22 - 32%[1] |
| Metabolism | Liver (CYP2D6) |
| Elimination half-life | 5-6 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.477 |
| Chemical and physical data | |
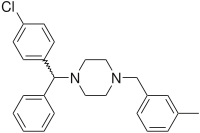
| Formula | C25H27ClN2 |
| Molar mass | 390.96 g·mol−1 |
| 3D model (JSmol) | |
| Boiling point | 230 °C (446 °F) |
| |
| |
| | |
Meclizine, sold under the brand name Bonine, among others, is an antihistamine used to treat motion sickness and dizziness (vertigo).[3] It is taken by mouth.[3] Effects generally begin in an hour and last for up to a day.[3]
Common side effects include sleepiness and dry mouth.[3] Serious side effects may include allergic reactions.[3] Use in pregnancy appears safe, but has not been well studied; use in breastfeeding is of unclear safety.[4] It is believed to work in part by anticholinergic and antihistamine mechanisms.[3]
Meclizine was patented in 1951 and came into medical use in 1953.[5] It is available as a generic medication and often over the counter.[3][6] In 2022, it was the 129th most commonly prescribed medication in the United States, with more than 4 million prescriptions.[7][8]
- ^ Sun J, Liu J, Zhang J, Xia H (June 2021). "Meclizine-loaded nanostructured lipid carriers to manage nausea and vomiting: Oral bioavailability improvement". Journal of Drug Delivery Science and Technology. 63: 102432. doi:10.1016/j.jddst.2021.102432.
- ^ Wang Z, Lee B, Pearce D, Qian S, Wang Y, Zhang Q, et al. (September 2012). "Meclizine metabolism and pharmacokinetics: formulation on its absorption". Journal of Clinical Pharmacology. 52 (9): 1343–1349. doi:10.1177/0091270011414575. PMID 21903894.
- ^ a b c d e f g "Meclizine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 22 March 2019.
- ^ "Meclizine Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 547. ISBN 9783527607495.
- ^ Cappa M, Cianfarani S, Ghizzoni L, Loche S, Maghnie M (2015). Advanced Therapies in Pediatric Endocrinology and Diabetology: Workshop, Rome, October 2014. Karger Medical and Scientific Publishers. p. 101. ISBN 9783318056372.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Meclizine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.