| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
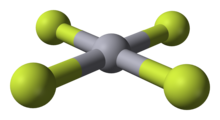
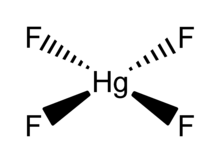
| HgF4 | |
| Molar mass | 276.58 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mercury(IV) fluoride, HgF4, is the first mercury compound to be reported with mercury in the +4 oxidation state. Mercury, like the other group 12 elements (cadmium and zinc), has an s2d10 electron configuration and generally only forms bonds involving its 6s orbital. This means that the highest oxidation state mercury normally attains is +2, and for this reason it is sometimes considered a post-transition metal instead of a transition metal. HgF4 was first reported from experiments in 2007, but its existence remains disputed; experiments conducted in 2008 could not replicate the compound.[1][2]
- ^ Is mercury a transition metal? Archived 2016-10-12 at the Wayback Machine
- ^ Rooms, John F.; Wilson, Antony V.; Harvey, Ian; Bridgeman, Adam J.; Young, Nigel A. (2008). "Mercury–fluorine interactions: a matrix isolation investigation of Hg···F2, HgF2 and HgF4 in argon matrices". Phys. Chem. Chem. Phys. 10 (31): 4594–4605. doi:10.1039/B805608K. PMID 18665309.