| |

| |
| Names | |
|---|---|
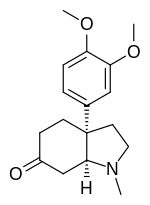
| IUPAC name
(3aS,7aS)-3a-(3,4-Dimethoxyphenyl)-1-methyl-2,3,4,5,7,7a-hexahydroindol-6-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H23NO3 | |
| Molar mass | 289.375 g·mol−1 |
| log P | 1.1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mesembrine is an alkaloid present in Sceletium tortuosum (kanna).[1] It has been shown to act as a serotonin reuptake inhibitor (Ki = 1.4 nM), and has also been found to behave as a weak inhibitor of the enzyme phosphodiesterase 4 (PDE4) (Ki = 7,800 nM).[2] In an in vitro study published in 2015, researchers concluded that "a high-mesembrine Sceletium extract" may exert anti-depressant effects by acting as a monoamine releasing agent."[3] As such, mesembrine likely plays a dominant role in the antidepressant effects of kanna.[4] The levorotatory isomer, (−)-mesembrine, is the natural form.[5]
Rat studies have evaluated effects of kanna extract, finding analgesic and antidepressant potential.[6] No adverse results were noted for a commercial extract up to 5000 mg/kg daily in rats.[7]
Mesembrine has also been identified in Mesembryanthemum cordifolium, Delosperma echinatum, and Oscularia deltoides.[8]
- ^ Smith, M. T.; Crouch, N. R.; Gericke, N.; Hirst, M. (March 1996). "Psychoactive constituents of the genus Sceletium N.E.Br. and other Mesembryanthemaceae: A Review". Journal of Ethnopharmacology. 50 (3): 119–130. doi:10.1016/0378-8741(95)01342-3. PMID 8691846.
- ^ Harvey, A. L.; Young, L. C.; Viljoen, A. M.; Gericke, N. P. (October 2011). "Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids". Journal of Ethnopharmacology. 137 (3): 1124–1129. doi:10.1016/j.jep.2011.07.035. PMID 21798331.
- ^ Coetzee, D. D.; López, V.; Smith, C. (November 2015). "High-mesembrine Sceletium extract (Trimesemine™) is a monoamine releasing agent, rather than only a selective serotonin reuptake inhibitor". Journal of Ethnopharmacology. 177: 111–116. doi:10.1016/j.jep.2015.11.034. PMID 26615766.
- ^ Stafford, G. I.; Pedersen, M. E.; van Staden, J.; Jäger, A. K. (October 2008). "Review on plants with CNS-effects used in traditional South African medicine against mental diseases". Journal of Ethnopharmacology. 119 (3): 513–537. doi:10.1016/j.jep.2008.08.010. PMID 18775771.
- ^ Coggon, P.; Farrier, D.S.; Jeffs, P.W.; McPhail, A.T. (1970). "Absolute configuration of mesembrine and related alkaloids: X-ray analysis of 6-epimesembranol methiodide". J. Chem. Soc. B: 1267–1271. doi:10.1039/J29700001267.
- ^ Loria, M. J.; Ali, Z; Abe, N; Sufka, K. J.; Khan, I. A. (Aug 8, 2014). "Effects of Sceletium tortuosum in rats". J. Ethnopharmacol. 155 (1): 731–5. doi:10.1016/j.jep.2014.06.007. PMID 24930358.
- ^ Murbach, T. S.; Hirka, G; Szakonyiné, I. P.; Gericke, N; Endres, J. R. (Dec 2014). "A toxicological safety assessment of a standardized extract of Sceletium tortuosum (Zembrin(®)) in rats". Food Chem. Toxicol. 74: 190–9. doi:10.1016/j.fct.2014.09.017. PMID 25301237.
- ^ Smith, Michael T.; Field, Courtney R.; Crouch, Neil R.; Hirst, Manton (January 1998). "The Distribution of Mesembrine Alkaloids in Selected Taxa of the Mesembryanthemaceae and their Modification in the Sceletium Derived 'Kougoed'". Pharmaceutical Biology. 36 (3): 173–179. doi:10.1076/phbi.36.3.173.6350.